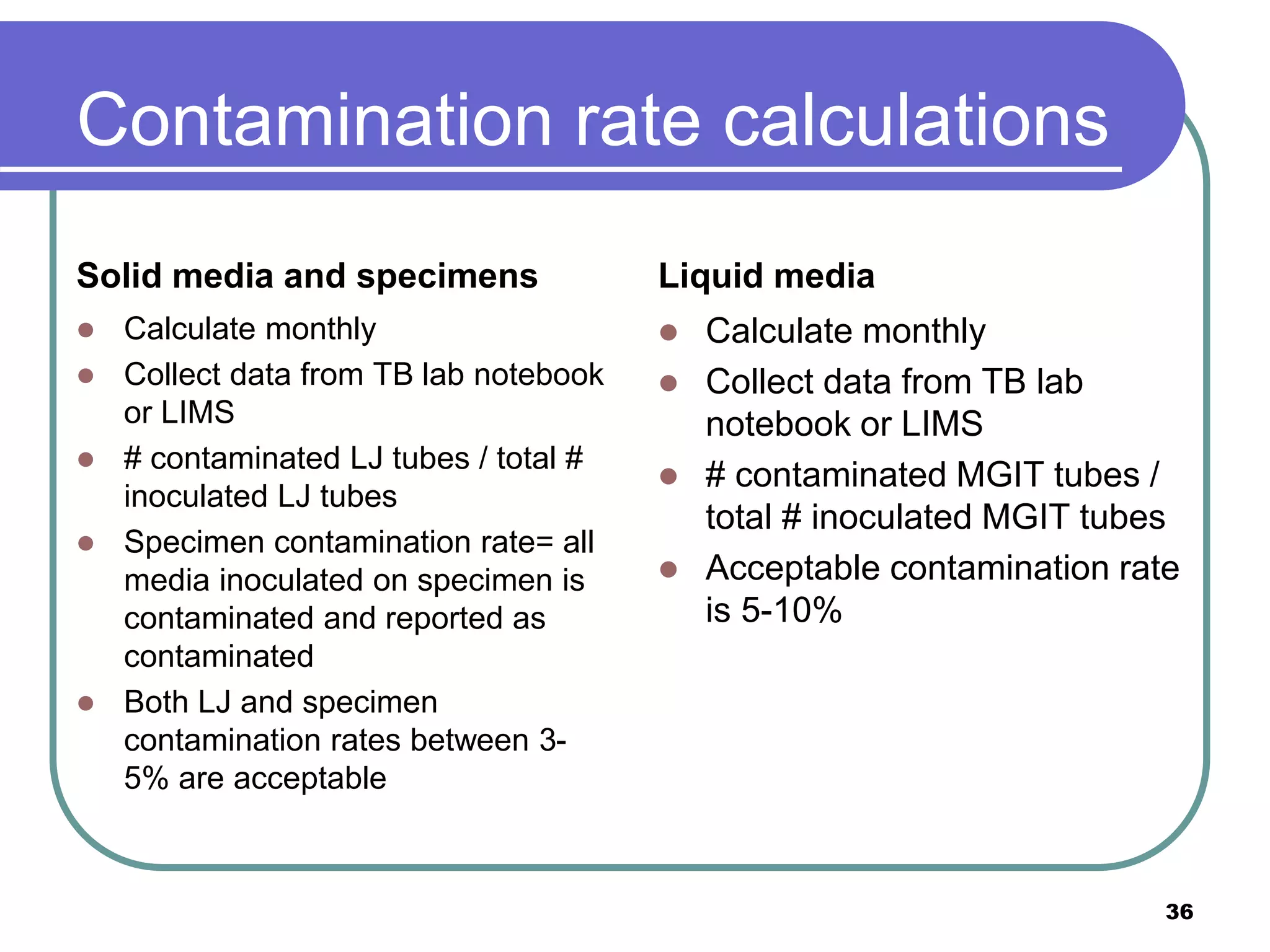
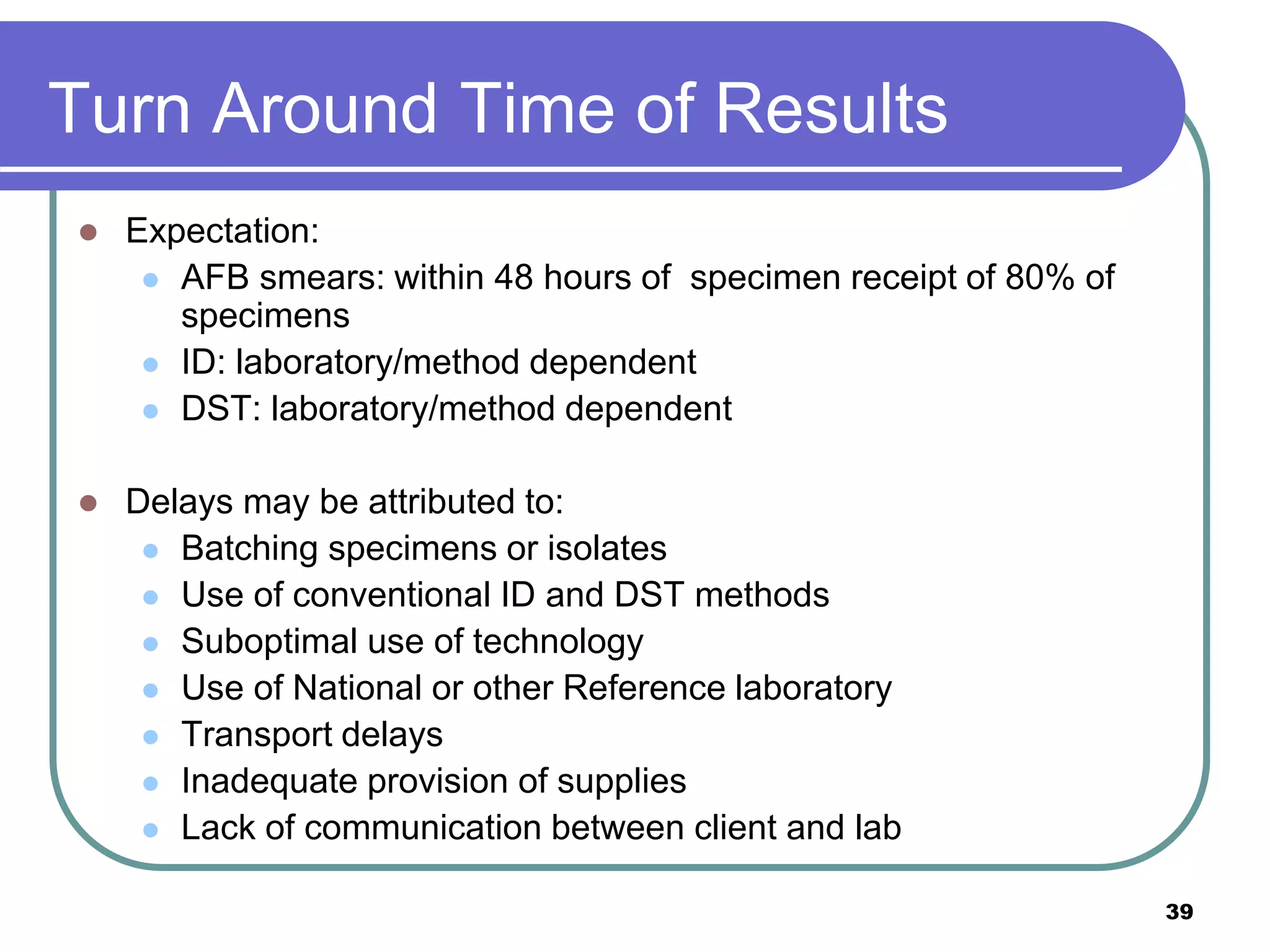
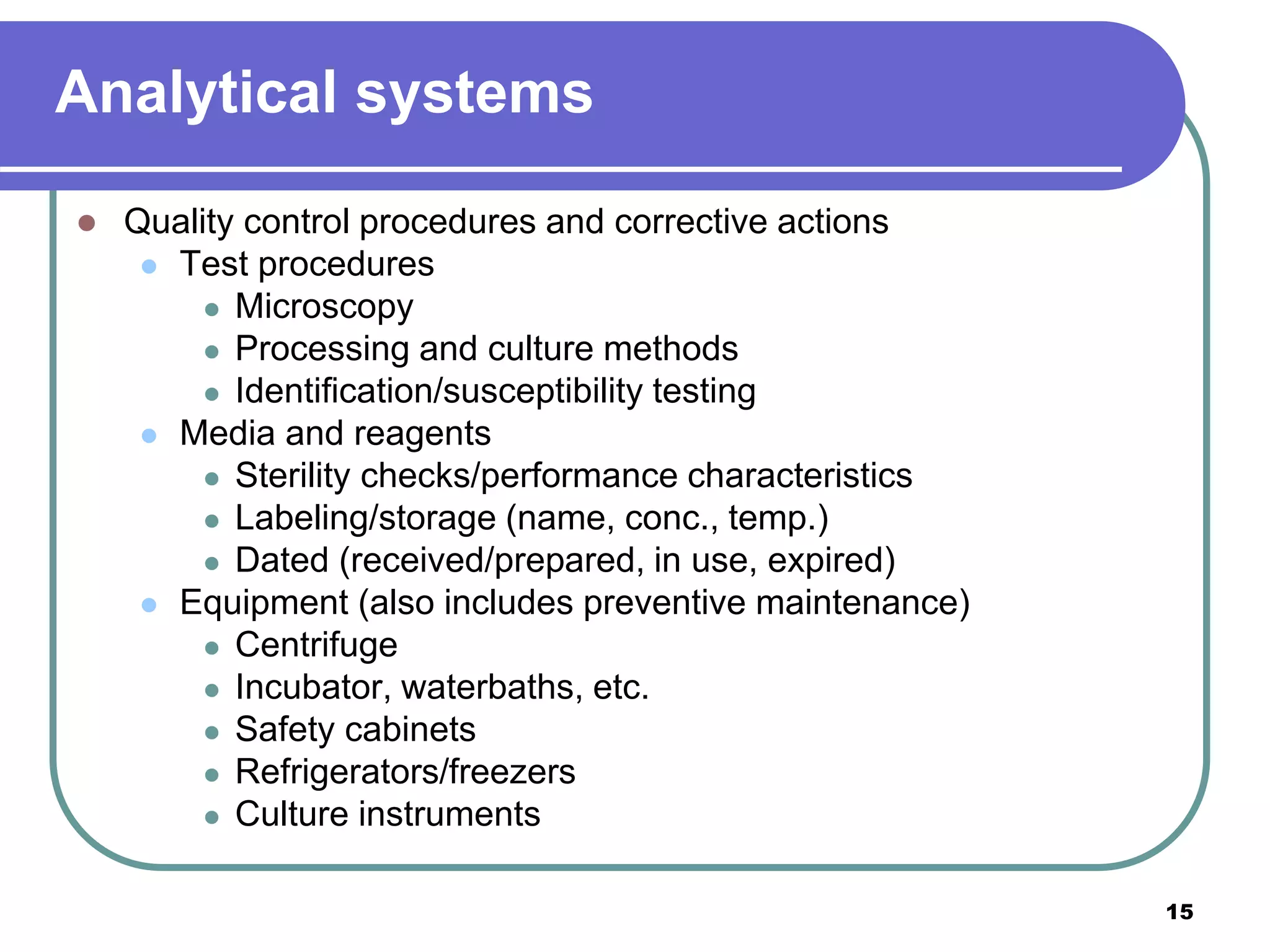
This document discusses quality assurance in mycobacteriology laboratories. It describes the three main components of a quality assurance system as quality control, external quality assessment, and quality improvement. Quality control procedures should address pre-analytical, analytical, and post-analytical phases of testing. Monitoring performance indicators such as contamination rates, turnaround times, and proficiency testing scores helps to evaluate laboratory performance and identify areas for improvement.














![Corrective actions: example
Identify (potential) problem: suboptimal or poor quality media [LJ]
Analyze the (potential) problem: poor quality media may impact the
yield/recovery of MTBC, lack of media may impact turn around time
Identify the (potential) solution: potential solutions include
purchasing commercially prepared media, or asking for assistance
from the NRL
Select and plan solution: gather catalog and vendor information or
contact NRL to establish relationship and determine the feasibility of
this option
Implement solution
Evaluate solution: determine the cost effectiveness of the solution,
the amount of lead time required to receive the media
Maintain and/or improve solution
Reference: Corrective and preventive action document. SANAS (South African
National Accreditation System)
18](https://image.slidesharecdn.com/qualityassurancequalindicators-230214063040-9523c2b5/75/Quality-Assurance-Qual-Indicators-ppt-18-2048.jpg)