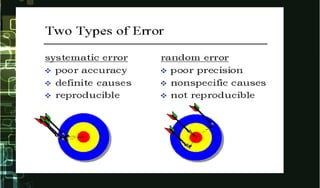
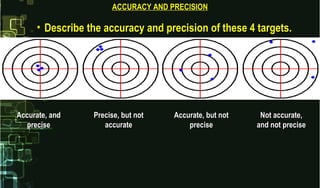
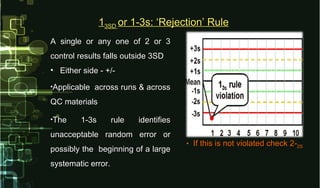
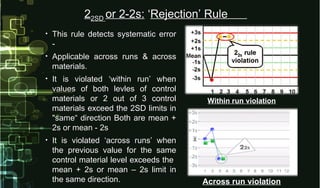
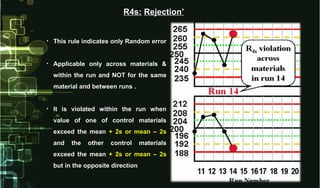
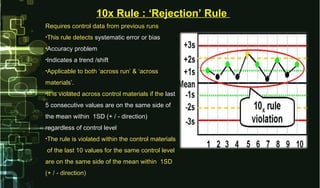
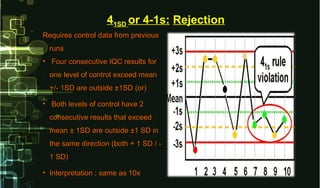
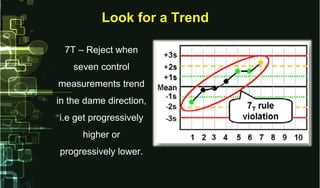
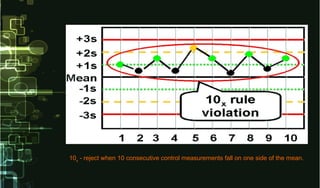
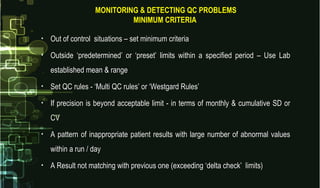
This document provides information on quality control and quality assurance in medical laboratories. It defines key terms like quality control, quality assurance, and quality assessment. It describes variables that can affect result quality and sources of errors. Random errors are unpredictable variations while systematic errors create biases. The document outlines Westgard rules, which are used to evaluate analytical runs and detect random and systematic errors. Steps for resolving quality control problems and minimum criteria for determining when results are out of control are also discussed.