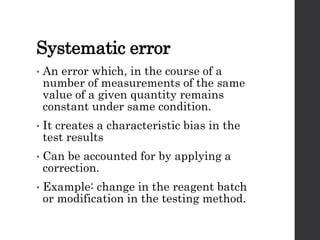
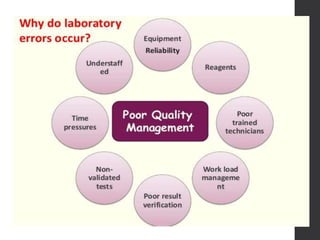
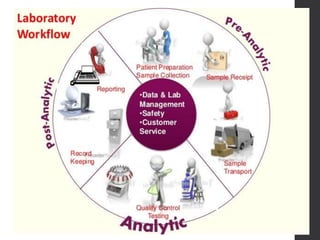
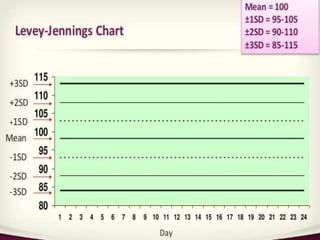
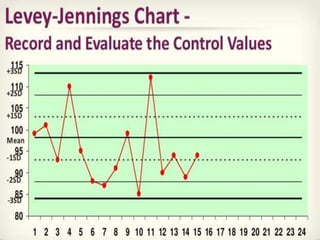
This document provides an overview of quality control in clinical biochemistry laboratories. It discusses that quality control aims to ensure test results are correct by minimizing errors. Errors can occur in the pre-analytical, analytical, and post-analytical phases. The pre-analytical phase, involving sample collection and handling, accounts for most errors. Laboratories use internal quality control methods like calibration, controls, and Levey-Jennings charts daily, as well as external quality assurance programs, to monitor performance and identify errors. Maintaining quality control is important for generating accurate, reliable test results.