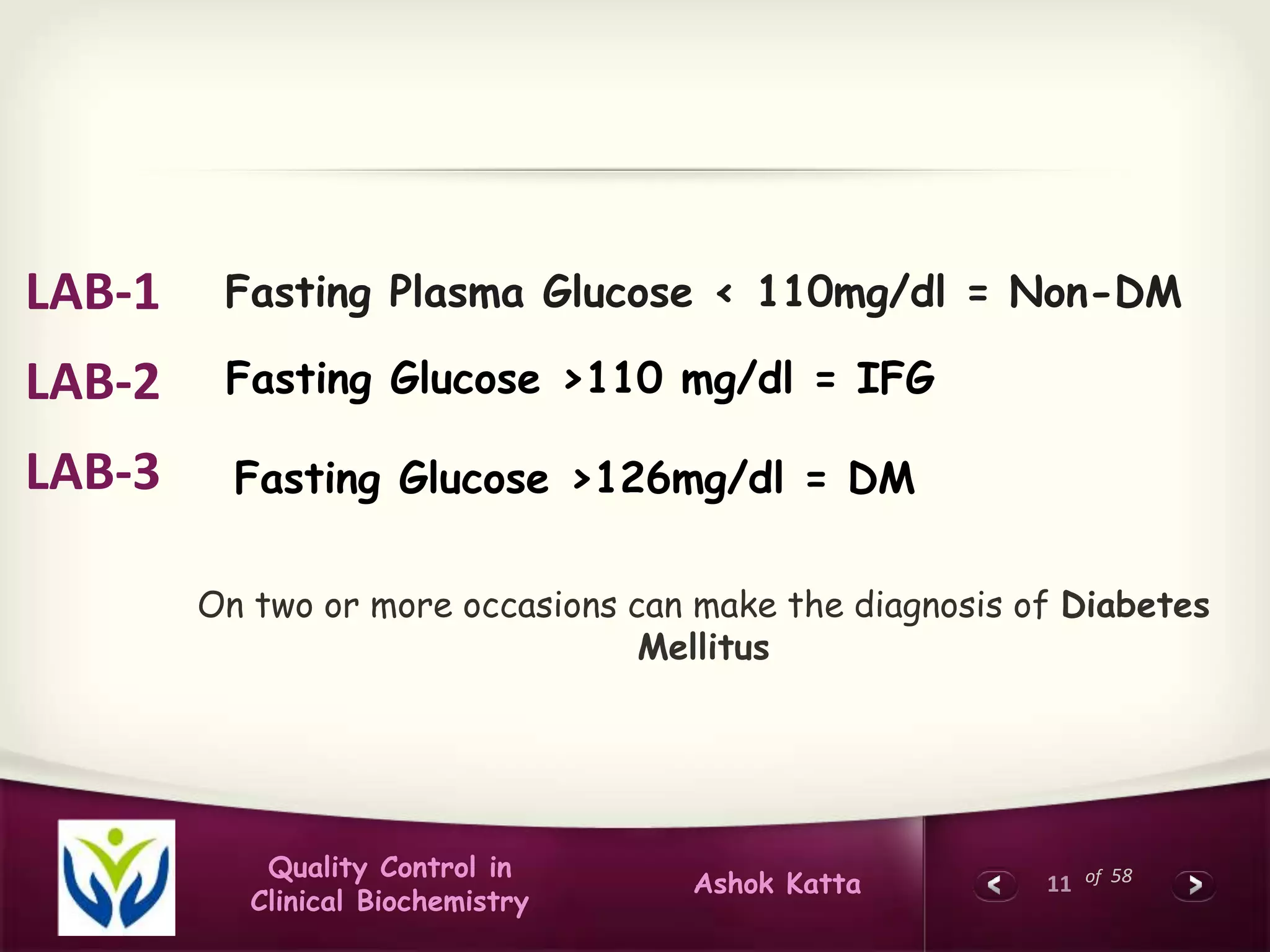
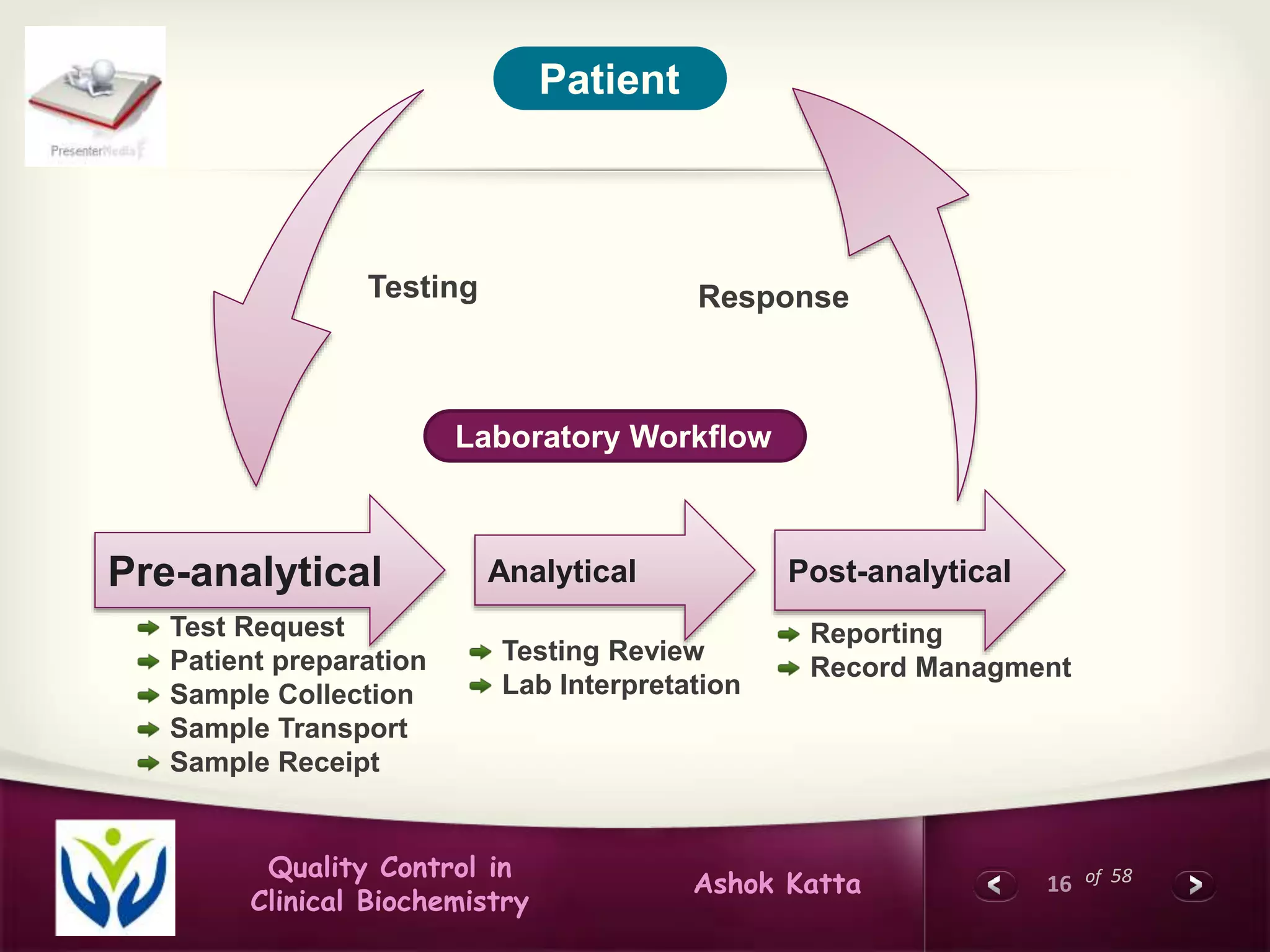
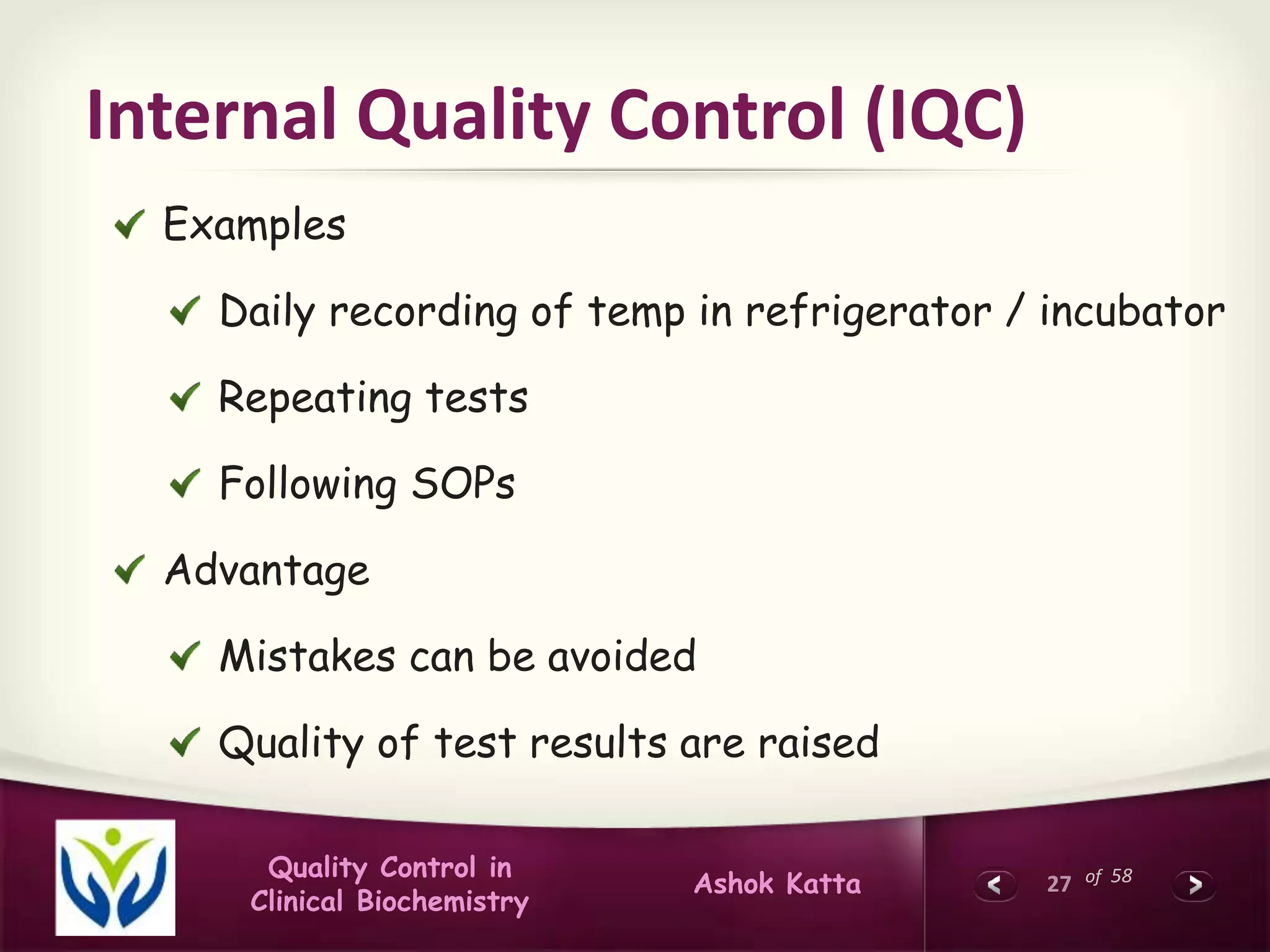
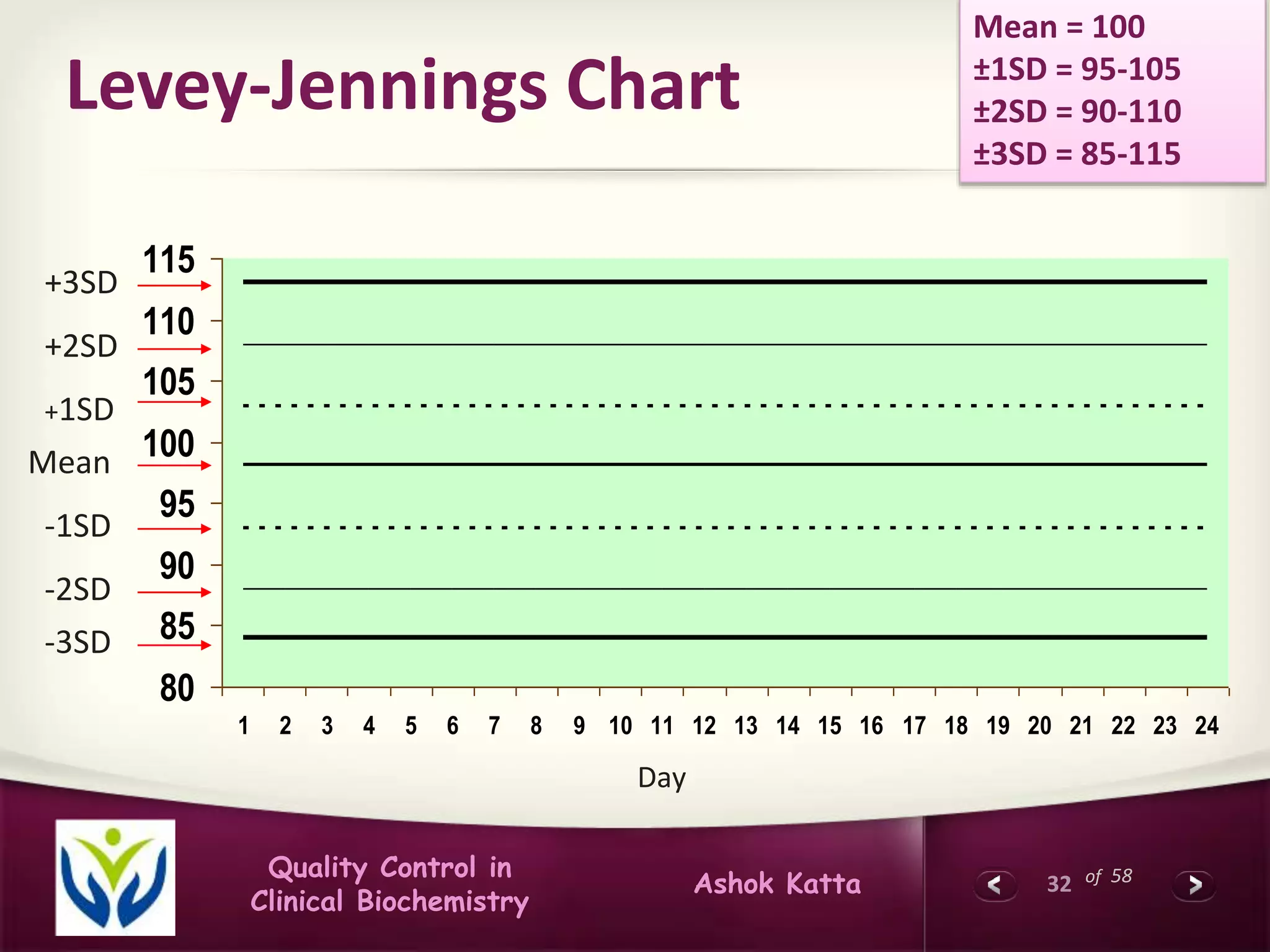
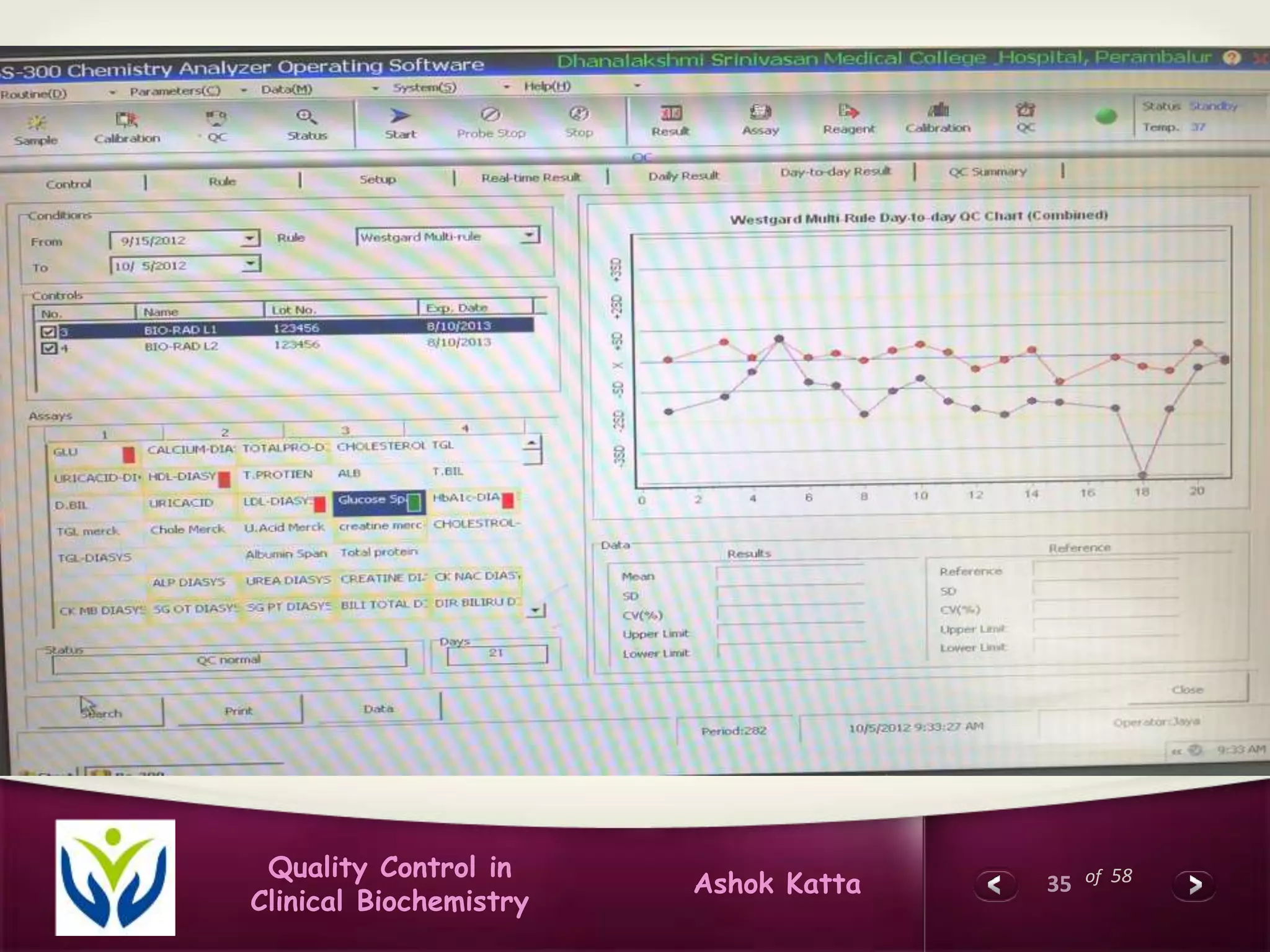
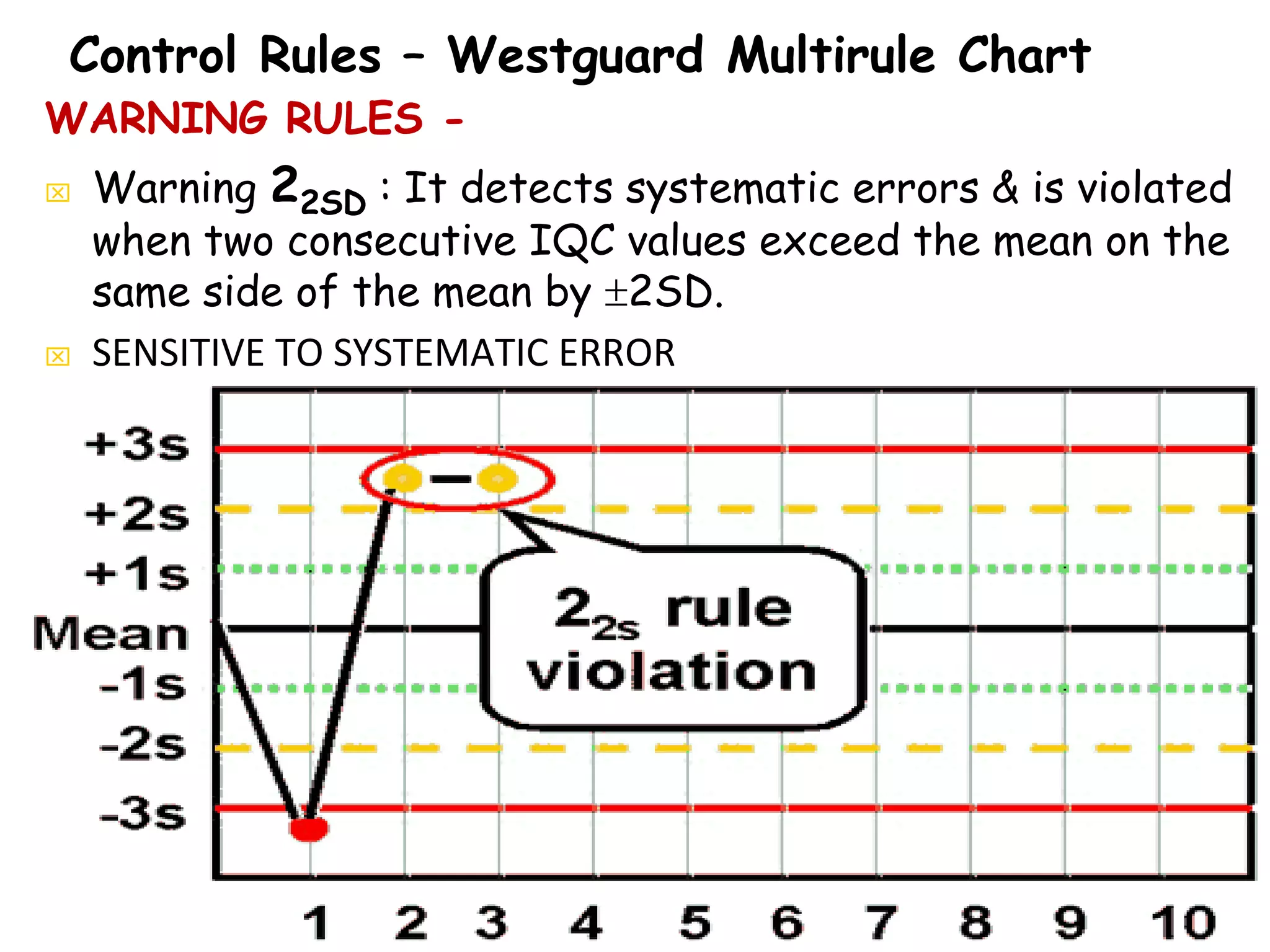
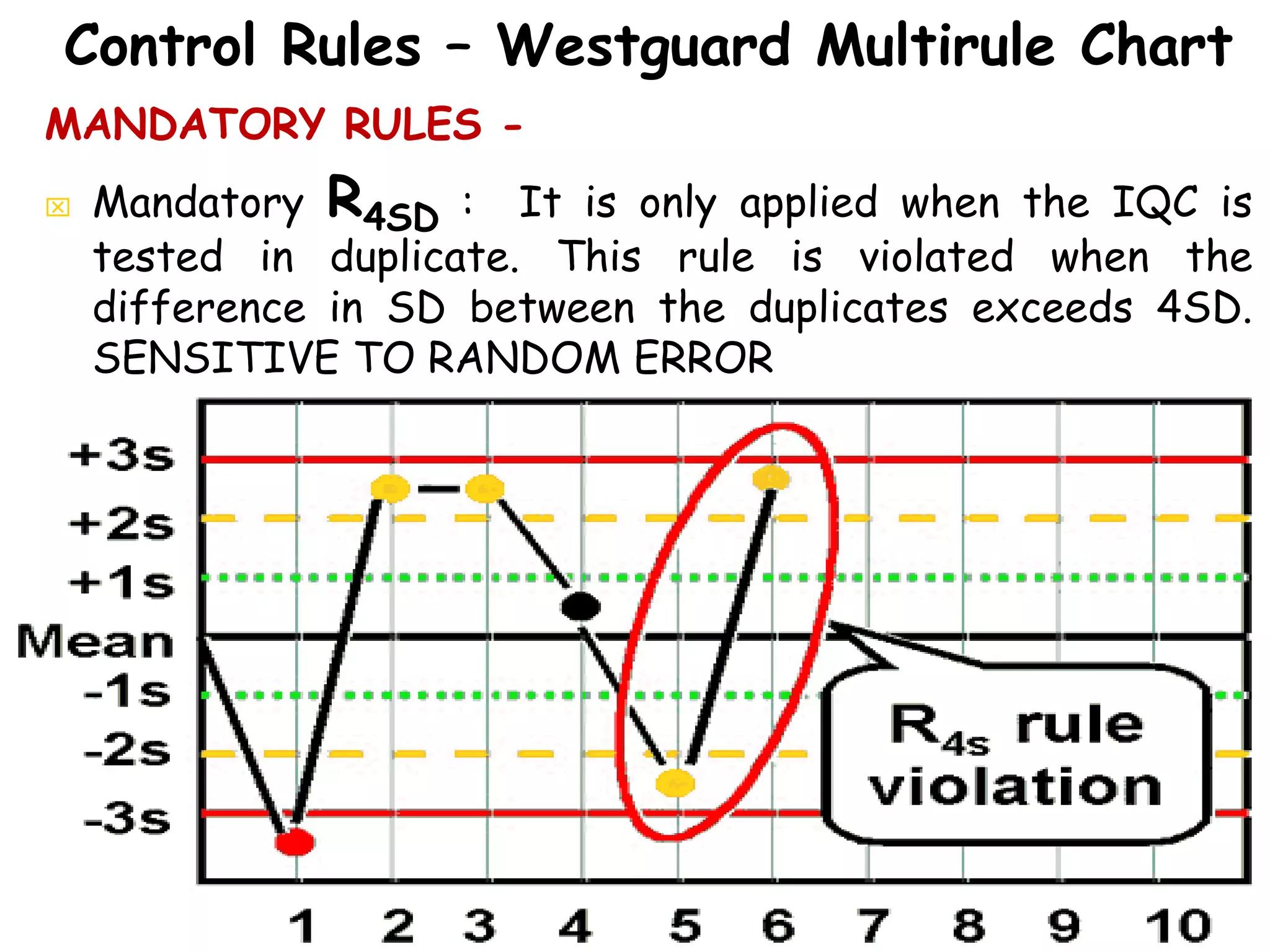
This document discusses quality control in clinical biochemistry laboratories. It explains that laboratory tests play an important role in clinical diagnosis and treatment decisions. Therefore, test results must be reliable and accurate. Quality control involves measures to ensure test accuracy, including internal quality control procedures done daily in the lab and external quality assessment involving evaluation by an outside agency. Proper quality control is essential to producing test results that healthcare providers can trust in making decisions for patients.







































![52 of 58Quality Control in
Clinical Biochemistry
Ashok Katta
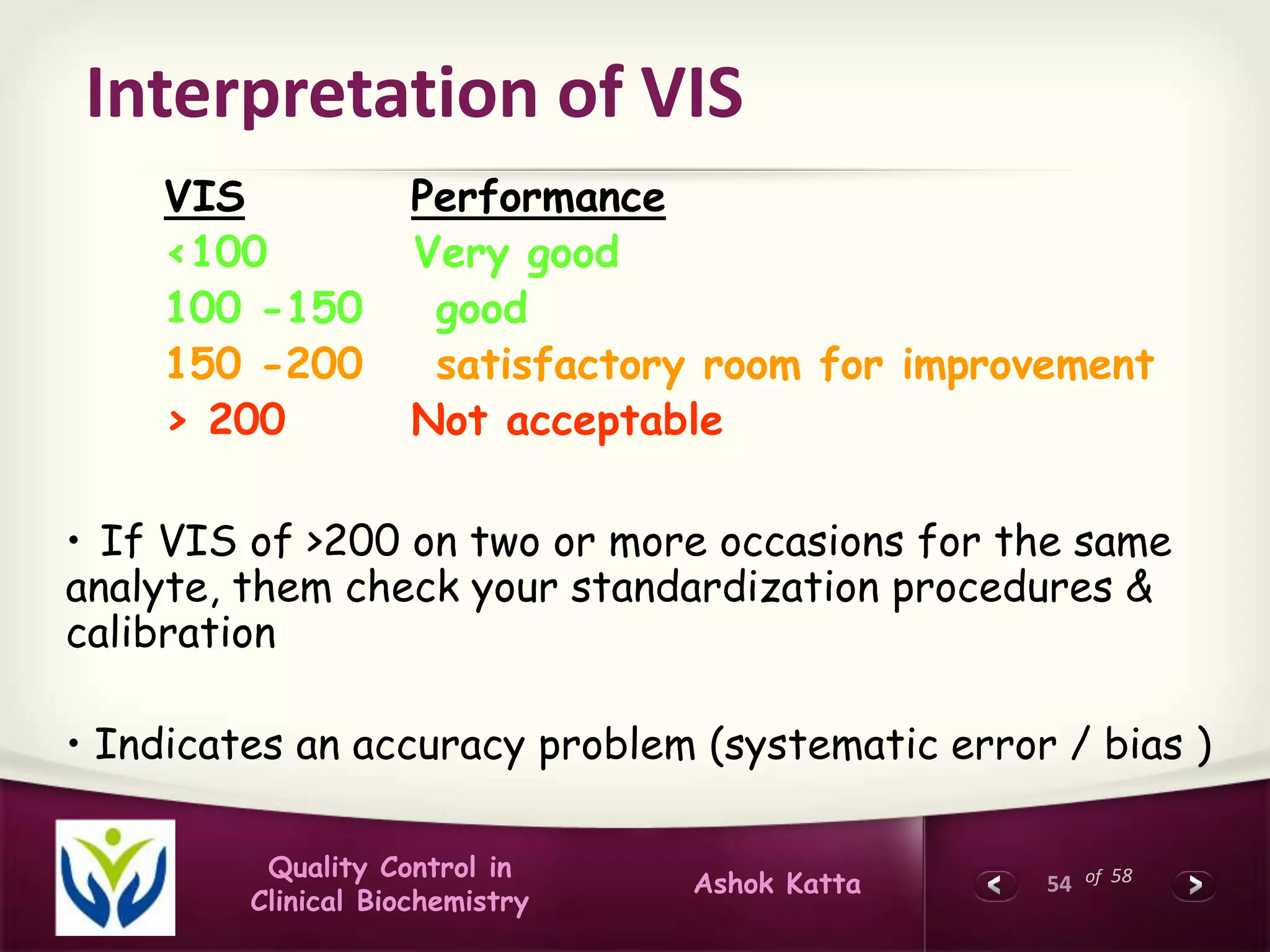
Calculation of VIS
Designated Value [DV] = 120 mg %
Participant's result = 95 mg%
% Variation [%V] = Participant's Result – [DV]
---------------------------------- X 100
Designated value
120-95 X 100 = 25 X 100
120 120
= 20.8
Variance
Index = %V X 100 = 20.8 X 100 = 277
CCV 7.5
VIS = 277](https://image.slidesharecdn.com/qualitycontrolinclinicalbiochemistry-141214074307-conversion-gate01/75/Quality-control-in-clinical-biochemistry-52-2048.jpg)