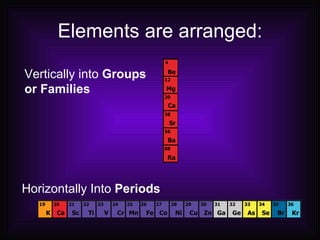
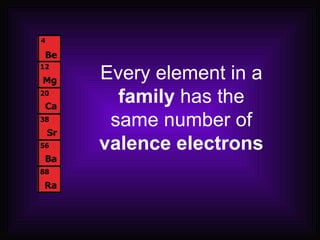
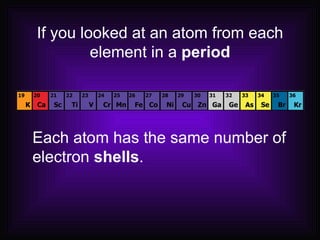
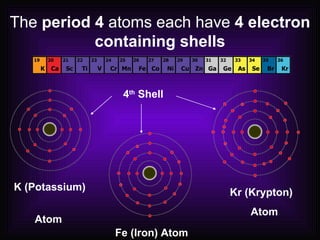
This document describes how elements are classified and organized on the periodic table. It defines metals, nonmetals, and metalloids, and explains their characteristic properties. Metals are shiny, solid conductors that are malleable and ductile, while nonmetals exhibit varying states, are dull, and are poor conductors. Metalloids have some properties of both. Elements are arranged vertically in groups with the same number of valence electrons and horizontally in periods with the same number of electron shells. The periodic law states that properties repeat periodically with increasing atomic number.