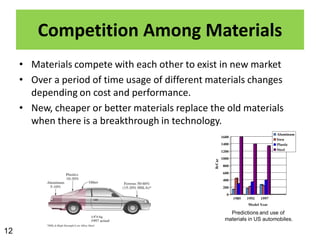
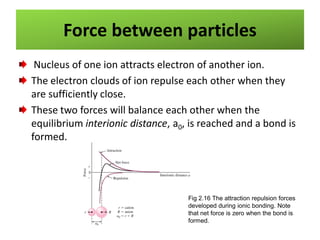
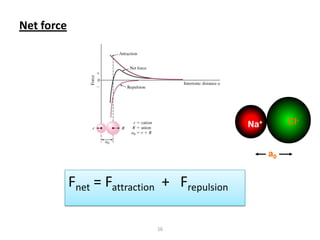
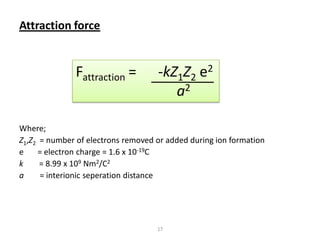
This document provides an overview of different types of materials including gases, condensed matter, metals, polymers, ceramics, composites, and electronics. It discusses the importance of materials science and engineering in areas like the economy, design, and new applications. The main classes of materials covered are metallic, polymeric, and ceramic materials. Composites combine two or more materials while maintaining a distinct boundary between them. Recent advances discussed include smart materials that react to stimuli and nanomaterials that have particle sizes smaller than 100 nm.