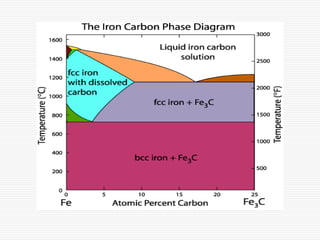
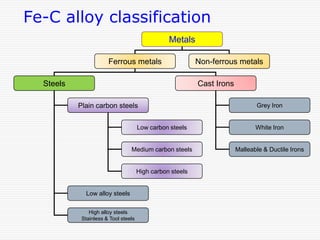
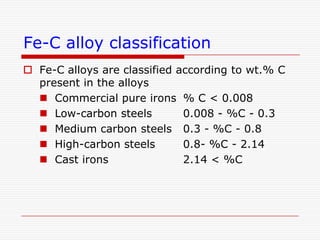
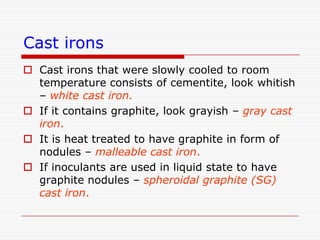
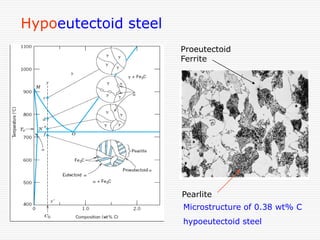
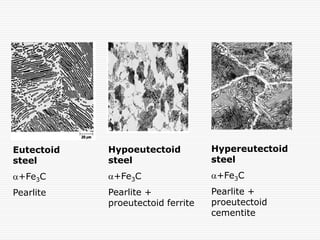
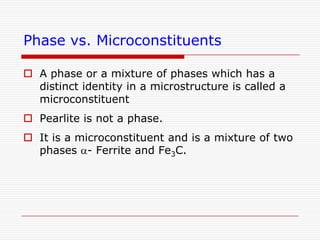
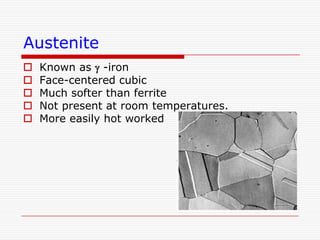
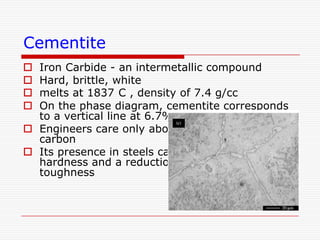
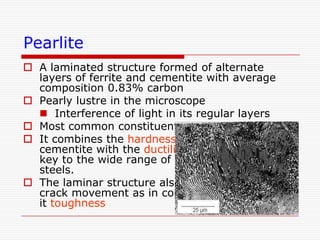
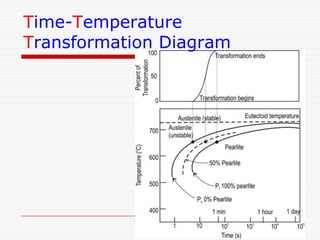
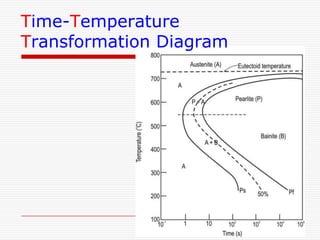
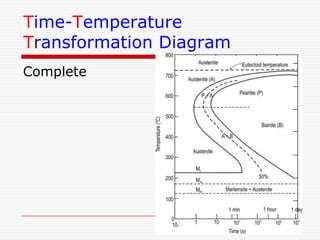
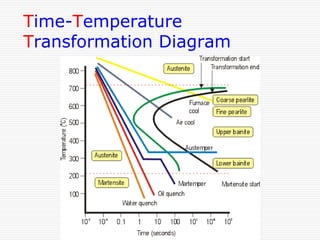
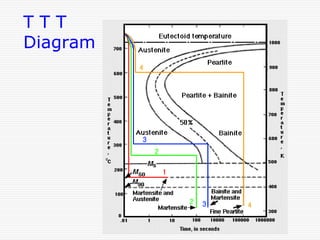
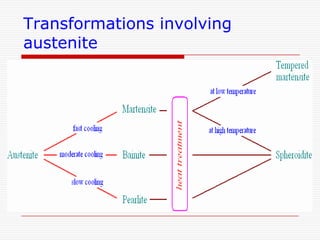
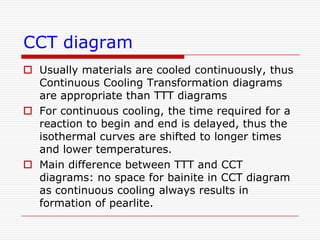
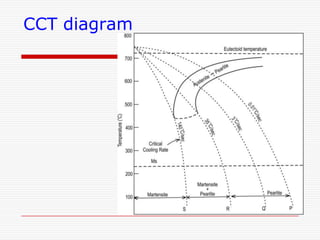
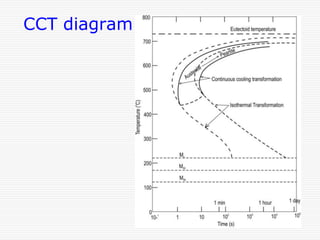
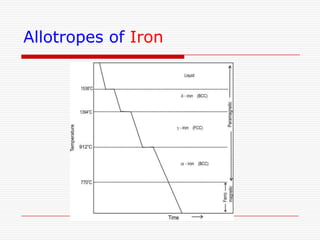
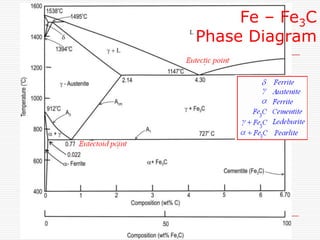
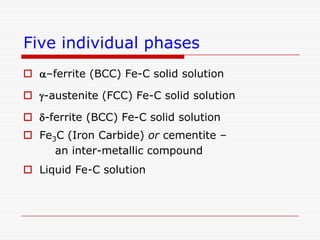
This document discusses metallurgy and material science, specifically focusing on the iron-carbon phase diagram and the microstructures and transformations associated with steels. It describes the five individual phases in the Fe-C diagram, including ferrite, austenite, cementite, and liquid. It also discusses the three invariant reactions of peritectic, eutectic, and eutectoid. The document classifies different types of steels and cast irons based on their carbon content and describes the microstructures of hypoeutectoid, eutectoid, and hypereutectoid steels. It also discusses phase transformations in steels including pearlite, bainite, and martensite


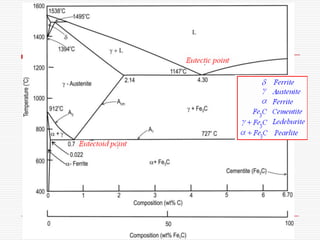
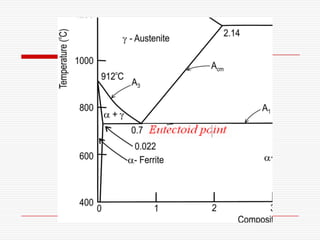
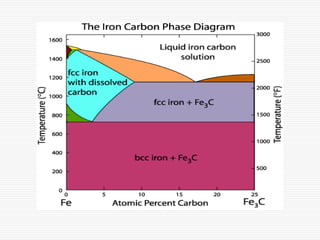
![Three invariant reactions
A horizontal line always indicates an invariant
reaction in binary phase diagrams
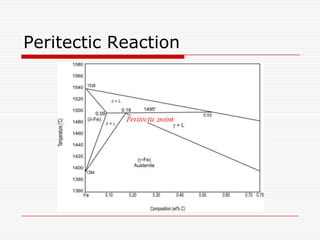
Peritectic reaction at 1495˚C and 0.18%C,
d-ferrite + L↔ g-iron (austenite)
Eutectic reaction at 1147˚C and 4.3 %C,
L ↔ g-iron + Fe3C (cementite) [ledeburite]
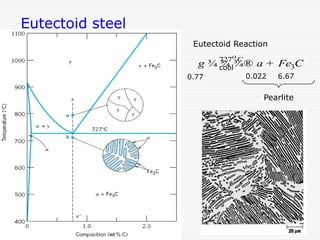
Eutectoid reaction at 727˚C and 0.77%C,
g-iron ↔ a–ferrite+Fe3C (cementite) [pearlite]](https://image.slidesharecdn.com/fe-cdiagram-140307110545-phpapp01/85/Iron-Carbon-Phase-Diagram-6-320.jpg)