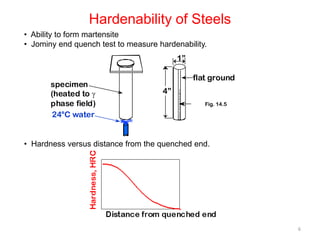
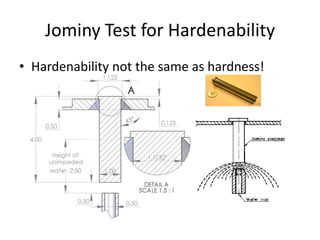
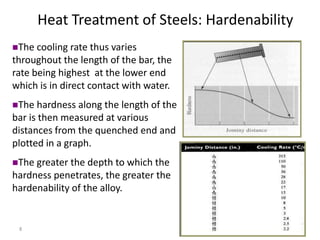
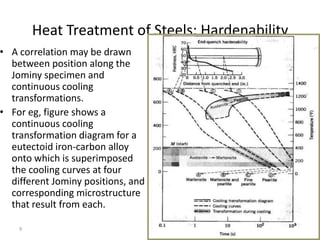
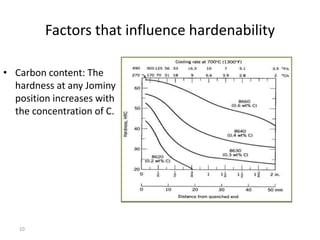
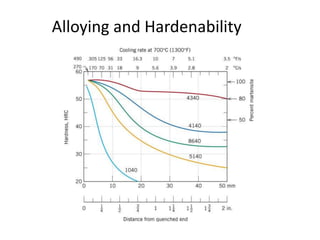
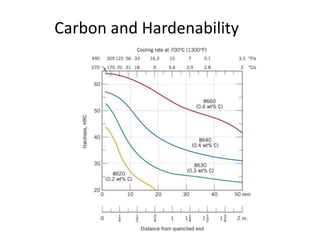
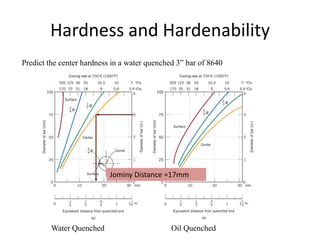
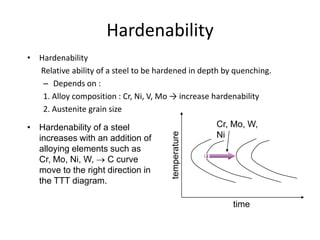
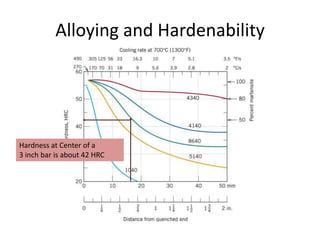
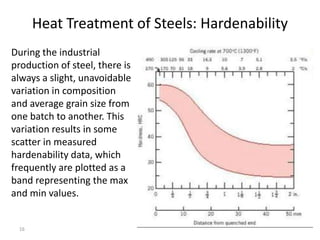
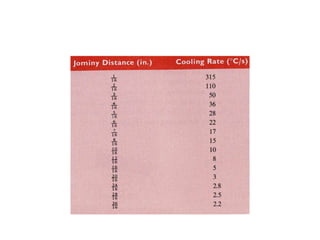
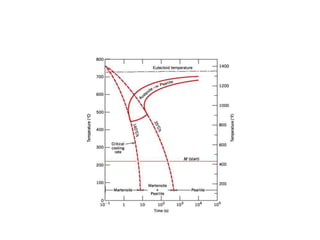
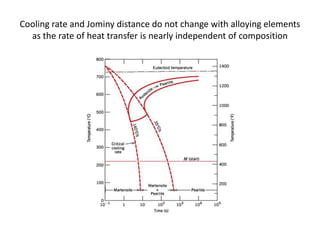
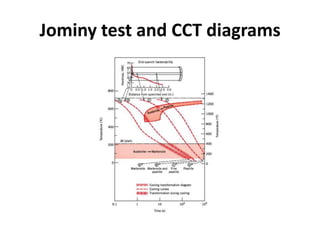
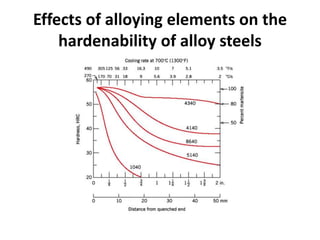
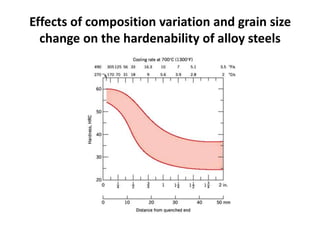
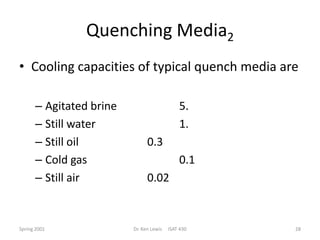
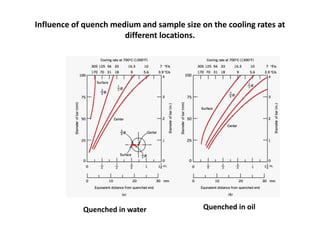
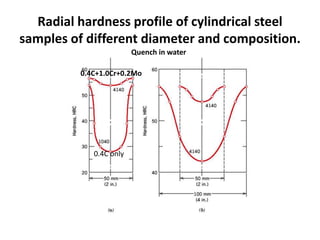
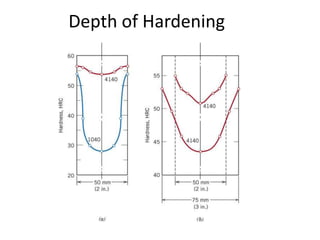
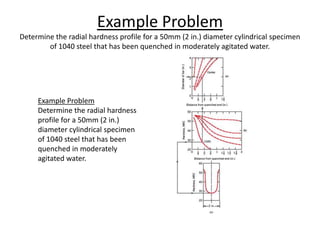
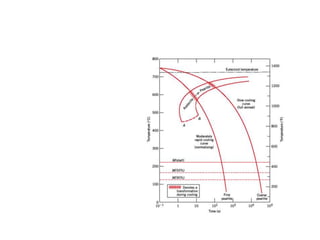
The document discusses hardenability, which is the ability of an alloy to form martensite and harden during heat treatment. It can be tested using the Jominy end-quench test, where a bar is heated and quenched at one end in water, causing a gradient of cooling rates and hardness levels along its length. Alloying elements like chromium, molybdenum, and nickel increase hardenability by shifting the CCT diagram to allow more martensite formation at a given cooling rate. The quenching medium, sample size, and alloy composition all impact the hardness profile achieved.