- Crystallographic points, directions and planes are specified using indexing schemes like Miller indices.
- Materials can be single crystals or polycrystalline aggregates of randomly oriented grains, leading to anisotropic or isotropic properties respectively.
- A crystal's diffraction pattern in reciprocal space is determined by its real space lattice and atomic structure. The reciprocal lattice is constructed geometrically from the real lattice and maps planes in real space to points in reciprocal space.
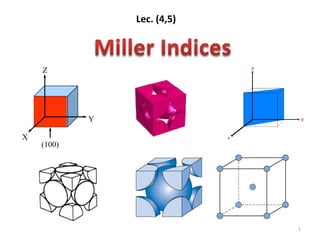

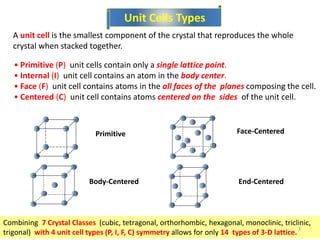


![ Miller indices - A shorthand notation to describe certain
crystallographic directions and planes in a material.
Denoted by [ ], <>, ( ) brackets. A negative number is
represented by a bar over the number.
Points, Directions and Planes in the
Unit Cell
6](https://image.slidesharecdn.com/phys4710lec45-150225030946-conversion-gate01/85/Miller-indecies-6-320.jpg)


![9
Crystallographic Directions
1. Vector repositioned (if necessary) to
pass through origin.
2. Read off projections in terms of
unit cell dimensions a, b, and c
3. Adjust to smallest integer values
4. Enclose in square brackets, no commas
[uvw]
ex: 1, 0, ½ => 2, 0, 1 => [201 ]
-1, 1, 1
z
x
where overbar represents a negative
index
[ 111]=>
y
[ 111]
[ 201]
Algorithm](https://image.slidesharecdn.com/phys4710lec45-150225030946-conversion-gate01/85/Miller-indecies-9-320.jpg)
![Procedure:
1. Any line (or vector direction) is specified by 2 points.
• The first point is, typically, at the origin (000).
2. Determine length of vector projection in each of 3 axes in
units (or fractions) of a, b, and c.
• X (a), Y(b), Z(c)
1 1 0
3. Multiply or divide by a common factor to reduce the
lengths to the smallest integer values, u v w.
4. Enclose in square brackets: [u v w]: [110] direction.
a
b
c
DIRECTIONS will help define PLANES (Miller Indices or plane normal).
[ 1 10]5. Designate negative numbers by a bar
• Pronounced “bar 1”, “bar 1”, “zero” direction.
6. “Family” of [110] directions is designated as <110>.
Directions in a Crystal
10](https://image.slidesharecdn.com/phys4710lec45-150225030946-conversion-gate01/85/Miller-indecies-10-320.jpg)
![Examples
210
X = ½ , Y = ½ , Z = 1
[½ ½ 1] [1 1 2]
X = 1 , Y = ½ , Z = 0
[1 ½ 0] [2 1 0]
11](https://image.slidesharecdn.com/phys4710lec45-150225030946-conversion-gate01/85/Miller-indecies-11-320.jpg)
![12
• When we write the direction
[n1n2n3] depending on the origin,
negative directions are written as
R = n1a1 + n2a2 + n3a3
To specify the direction, the
smallest possible integers must
be used.
Y direction
(origin) O
- Y direction
X direction
- X direction
Z direction
- Z direction
][ 321 nnn
Negative Directions
12](https://image.slidesharecdn.com/phys4710lec45-150225030946-conversion-gate01/85/Miller-indecies-12-320.jpg)
![13
X = -1 , Y = -1 , Z = 0 [110]
X = 1 , Y = 0 , Z = 0 [1 0 0]
Examples of Crystal Directions
13](https://image.slidesharecdn.com/phys4710lec45-150225030946-conversion-gate01/85/Miller-indecies-13-320.jpg)
![14
X =-1 , Y = 1 , Z = -1/6
[-1 1 -1/6] [6 6 1]
A vector can be moved to the origin.
Examples
14](https://image.slidesharecdn.com/phys4710lec45-150225030946-conversion-gate01/85/Miller-indecies-14-320.jpg)













![30
Miller Indices
Reciprocal numbers are:
2
1
,
2
1
,
3
1
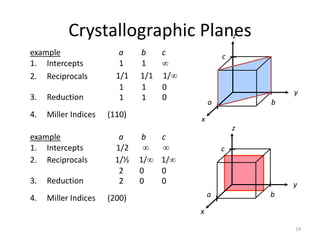
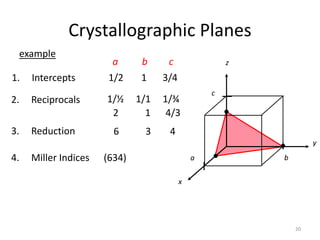
Plane intercepts axes at cba 2,2,3
Indices of the plane (Miller): (2,3,3)
(200) (100)
Indices of the direction: [2,3,3]a
3
2
2
b
c
[2,3,3]
Z
X
Y
(100)
Z
X
Y
(110)
Z
X
Y
(111)](https://image.slidesharecdn.com/phys4710lec45-150225030946-conversion-gate01/85/Miller-indecies-30-320.jpg)





