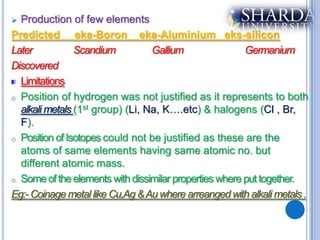
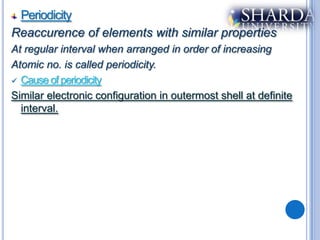
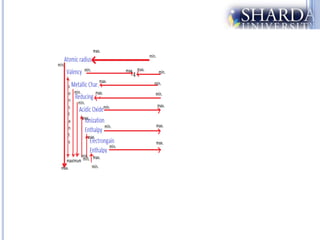
The document discusses periodic trends in properties such as atomic radius, ionization energy, and electronegativity. It explains Mendeleev's periodic table and how modern periodic law arranges elements based on atomic number instead of atomic mass. Periodicity in properties occurs because similar electronic configurations repeat at regular intervals as atomic number increases. Atomic radius generally decreases across a period as nuclear charge increases, and increases down a group as more shells are added. Ionization energy is the energy required to remove an electron from an atom, and is directly proportional to nuclear charge and inversely proportional to size and shielding effects.