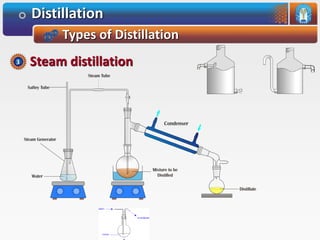
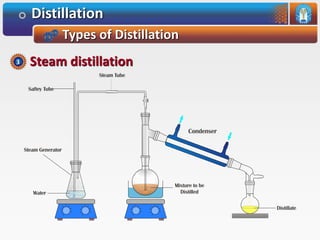
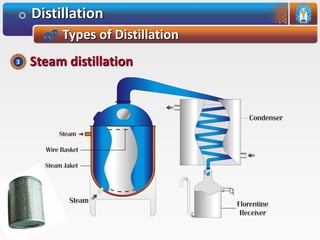
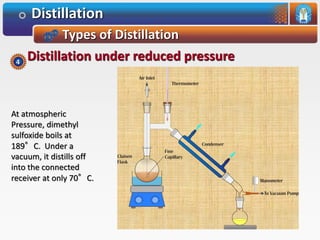
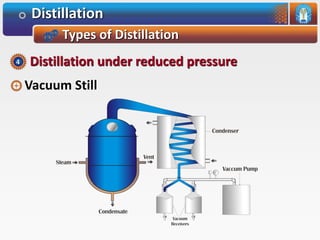
Distillation is a process used to separate mixtures based on differences in their boiling points. It involves heating the mixture until it vaporizes, then cooling the vapors until they condense. There are several types of distillation processes. Simple distillation is used to purify liquids. Fractional distillation separates mixtures with components of different boiling points. Steam distillation is used for mixtures containing water. Vacuum distillation allows distillation of substances that decompose at their normal boiling points. Destructive distillation involves decomposition during heating.











































![ Define the following terms:
[Distillation, etc]
Respond to the following questions:
Give a detailed account of ………………
Explain in details the process of …………..
Describe in details with examples the…………
With examples, illustrate the pharmaceutical applications of ……………](https://image.slidesharecdn.com/15-distillation-200630110940/85/15-distillation-52-320.jpg)