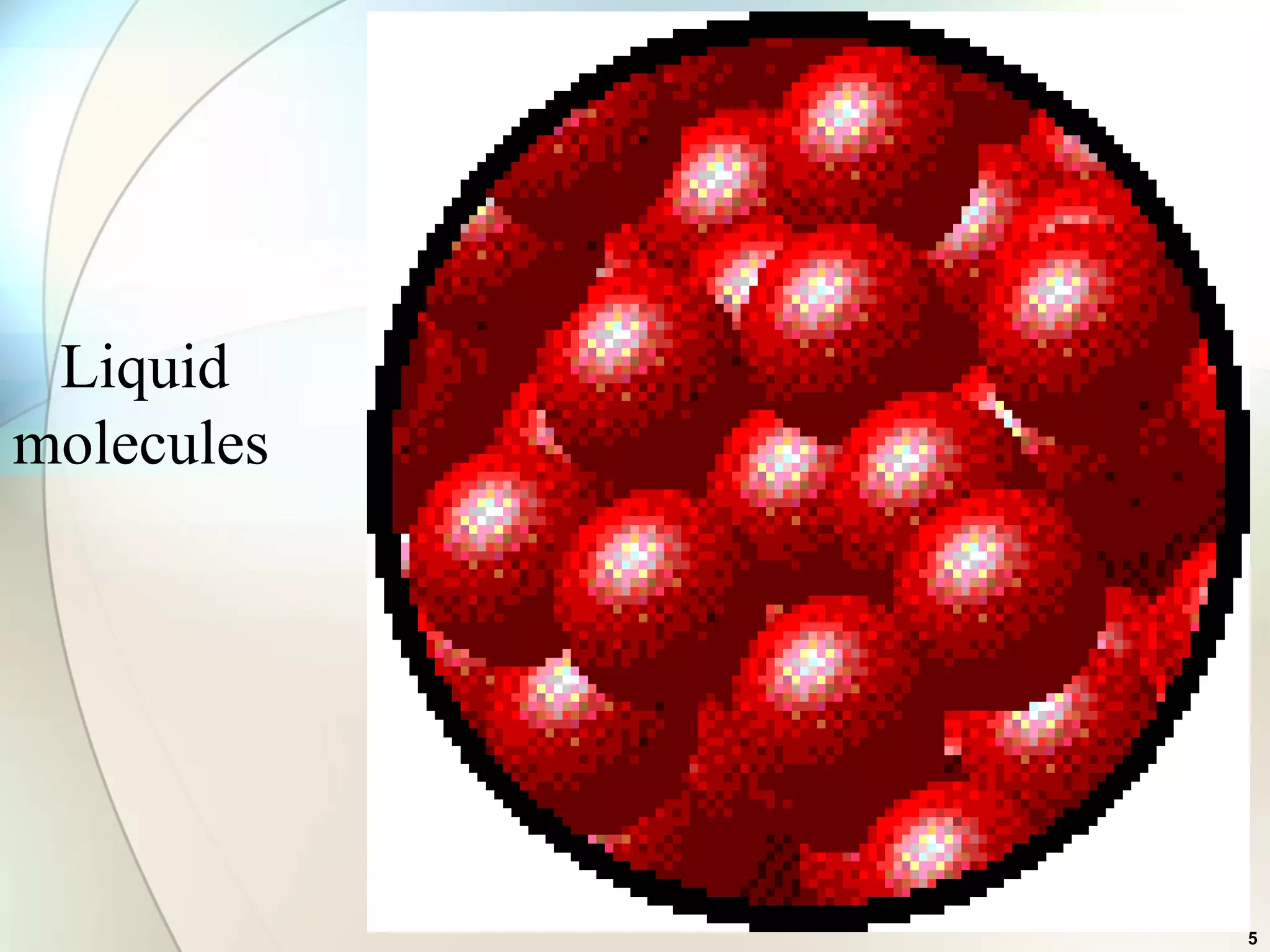
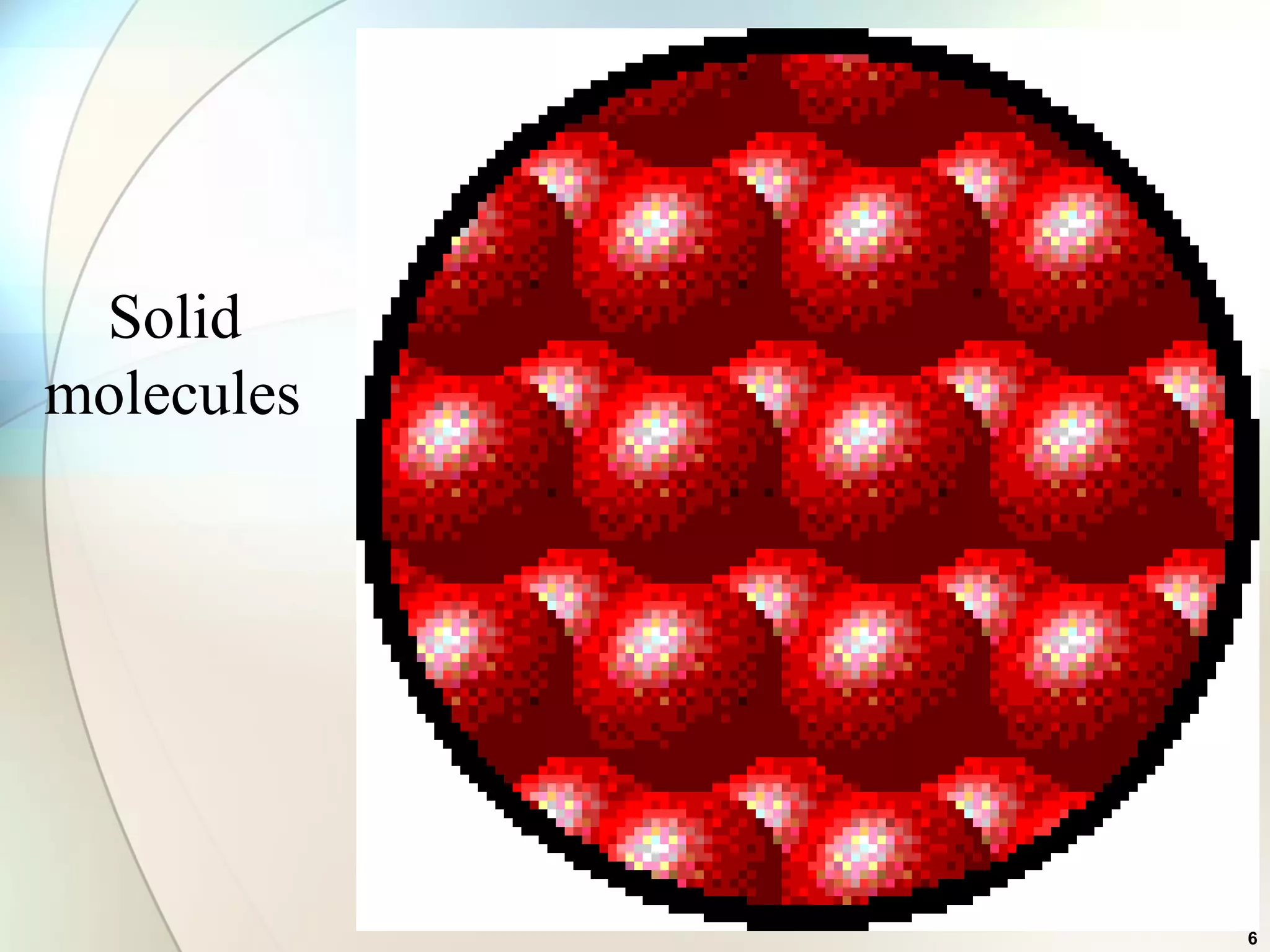
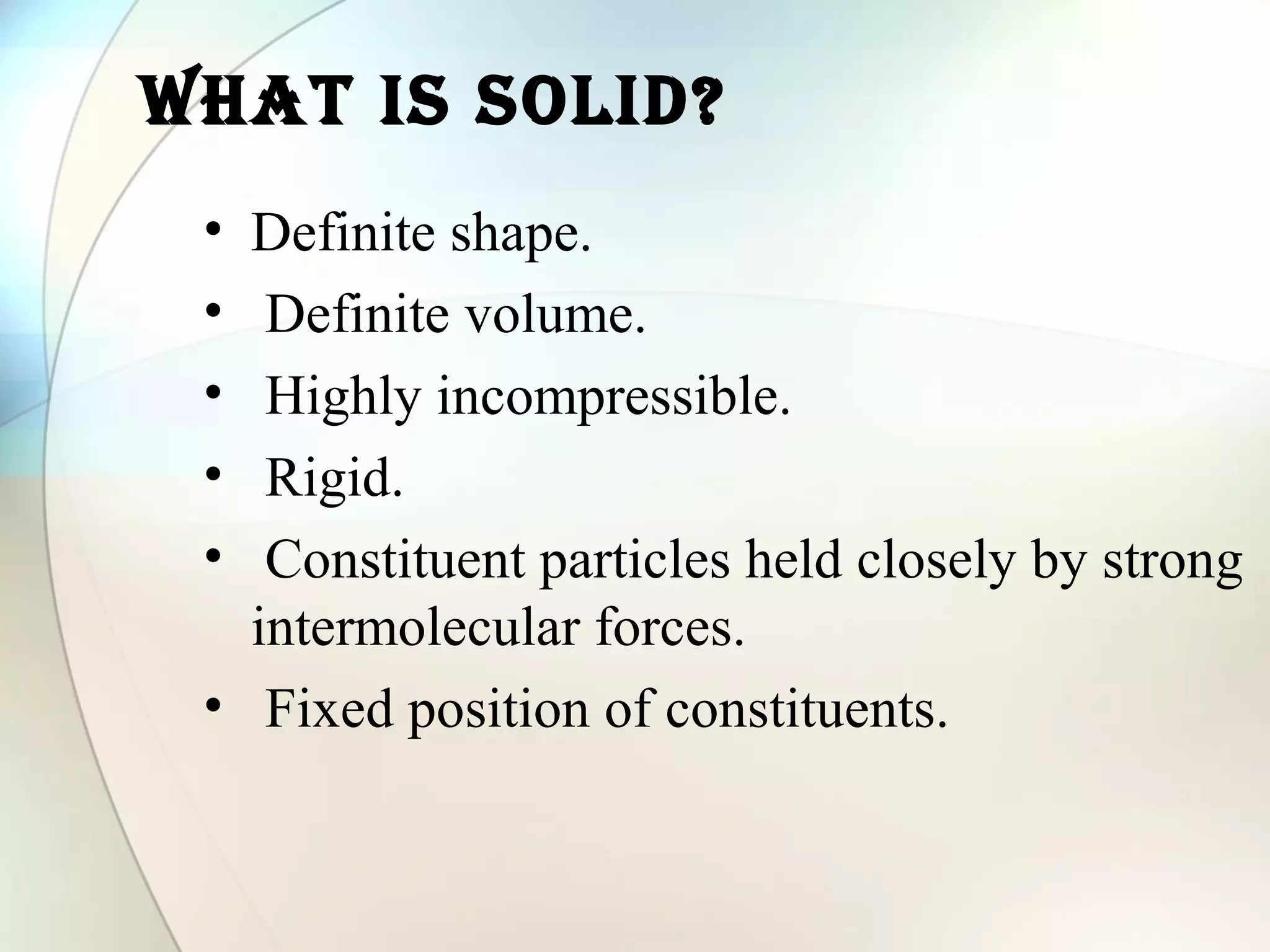
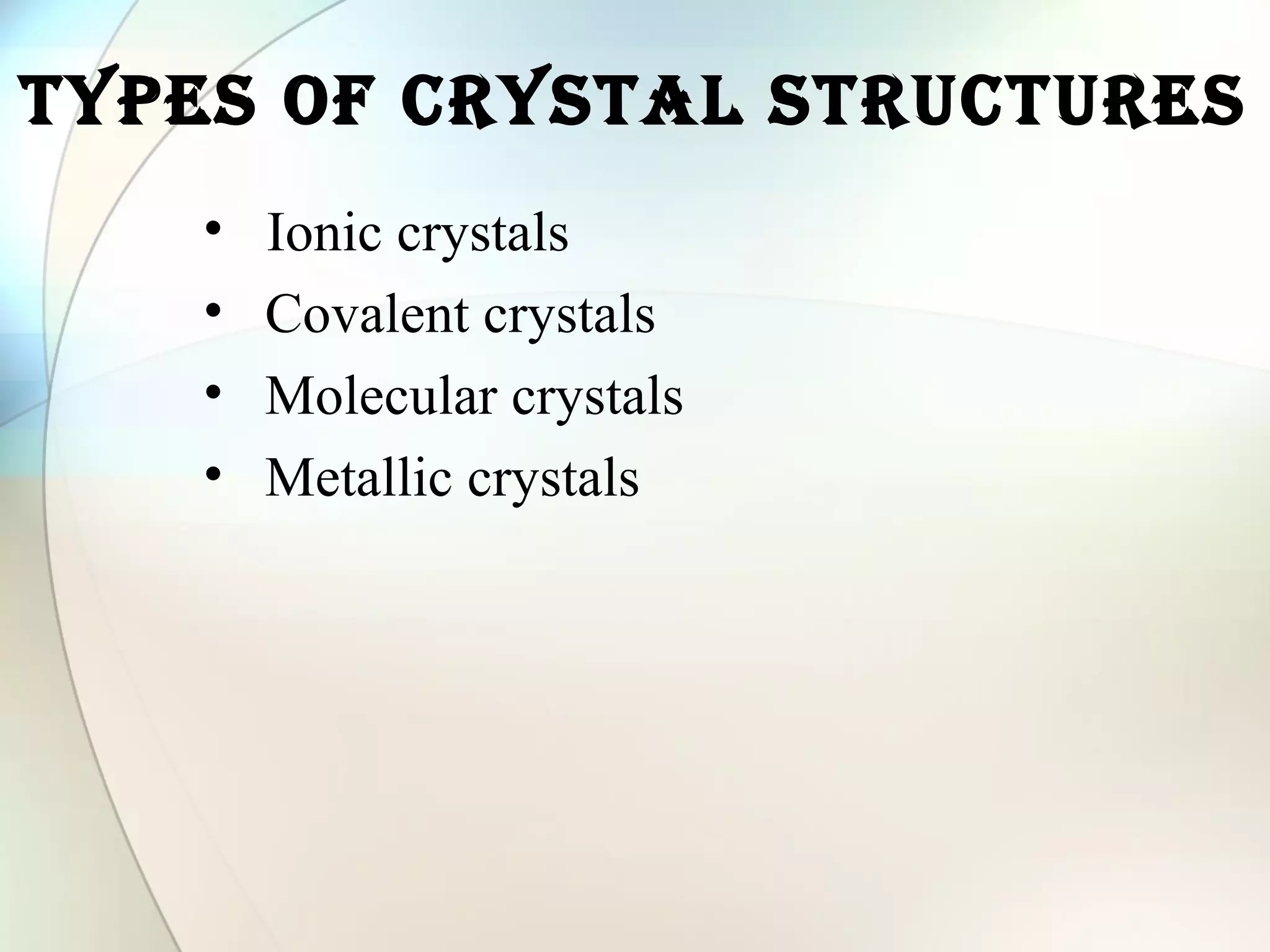
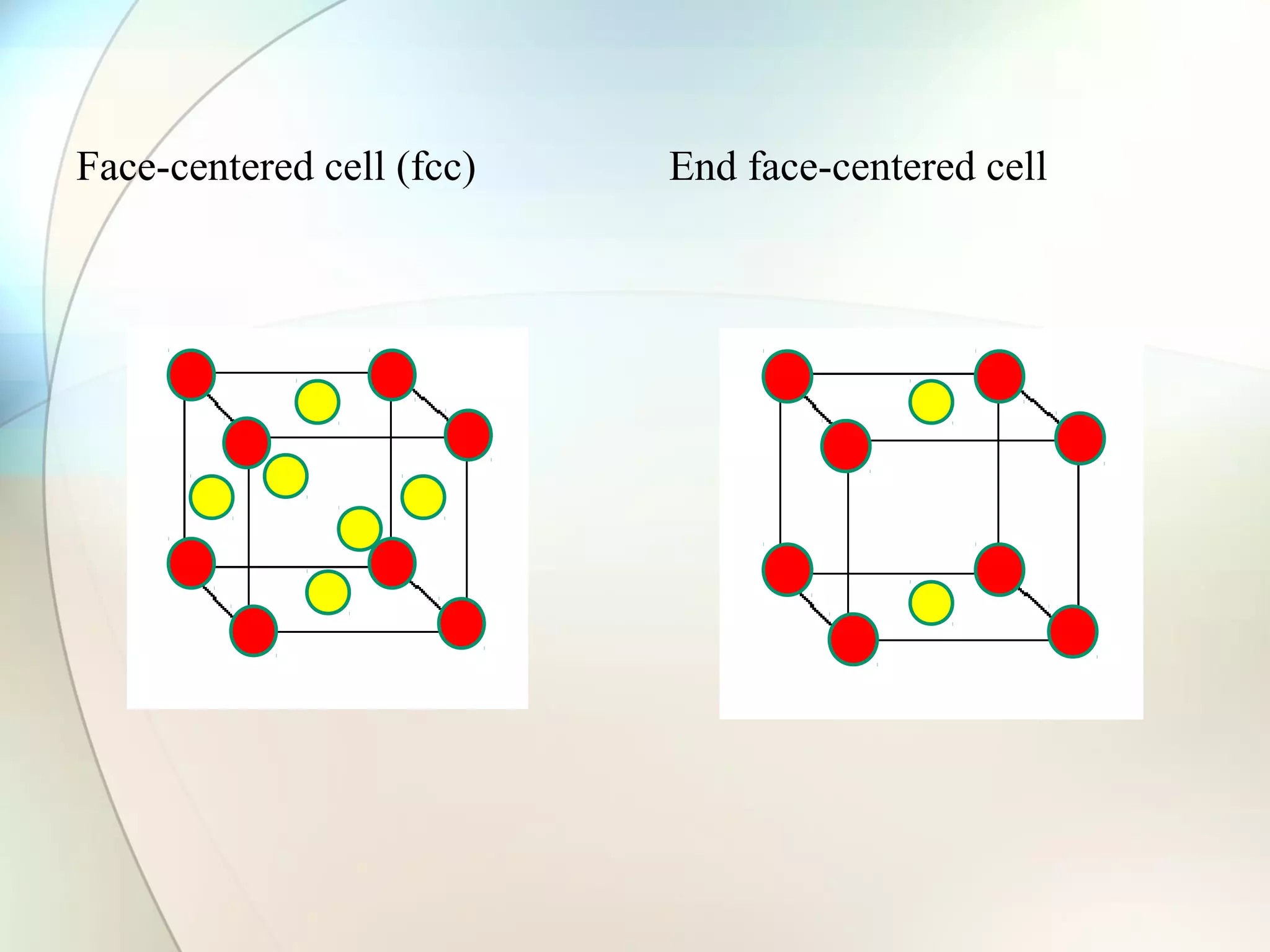
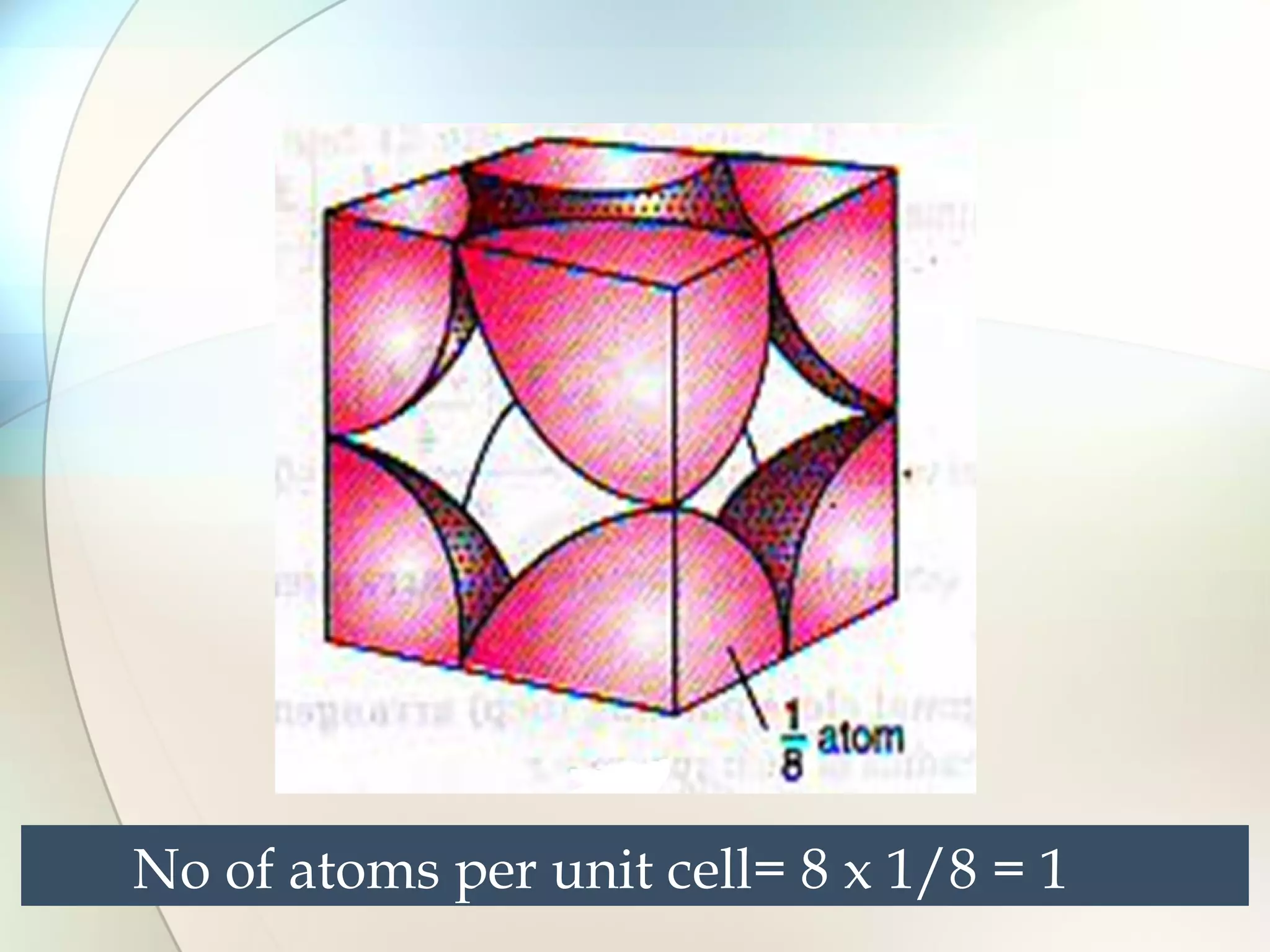
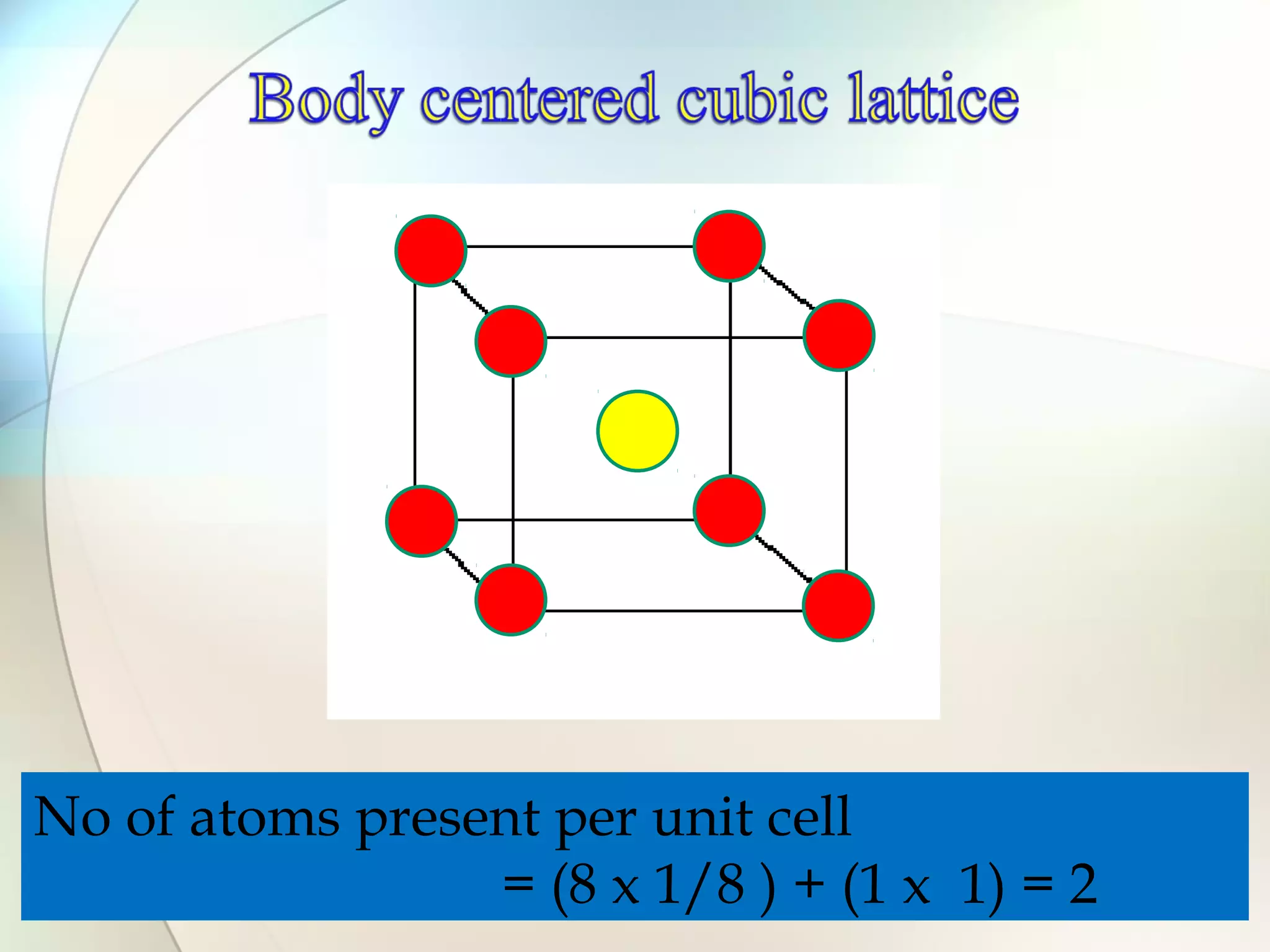
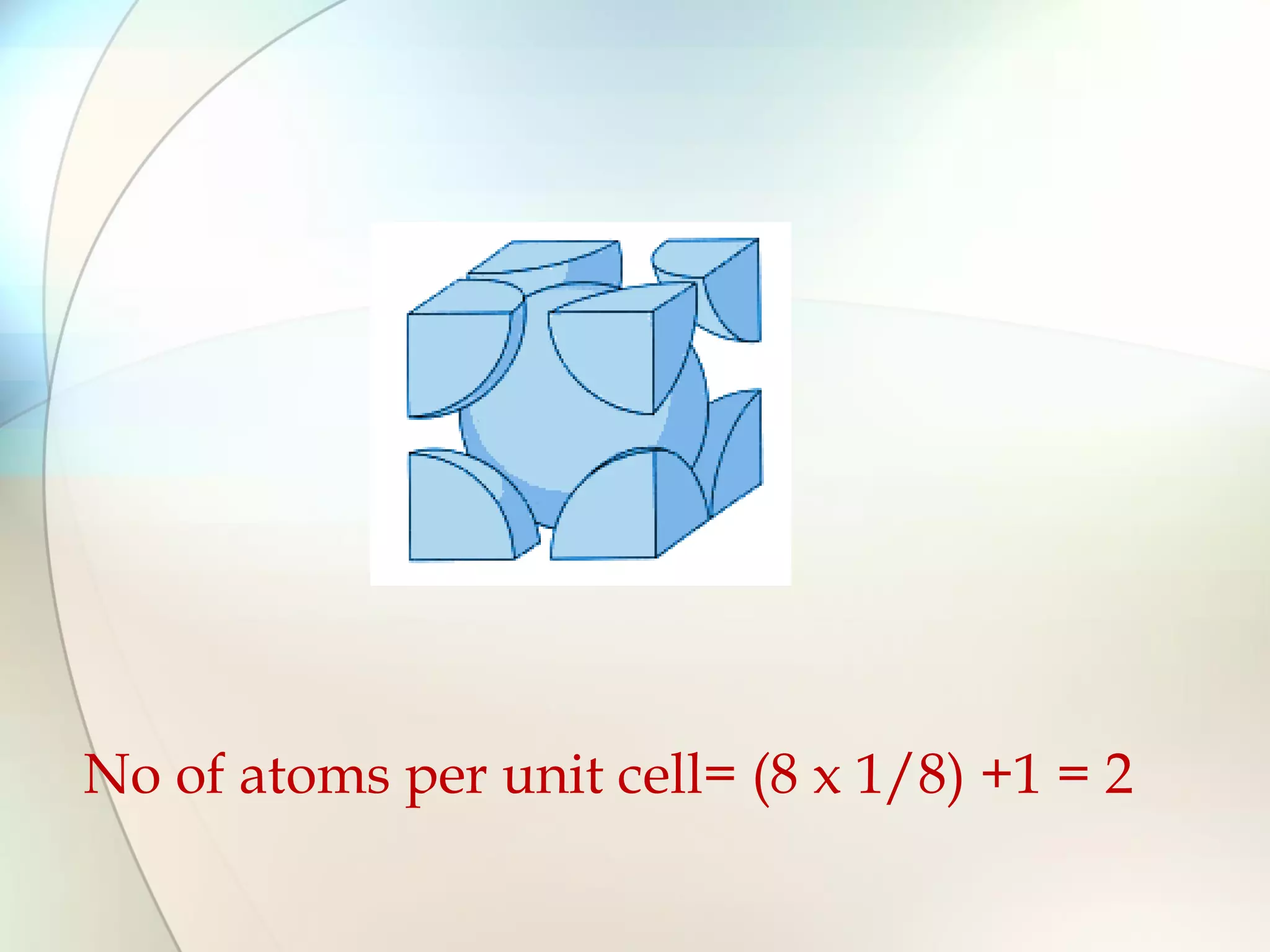
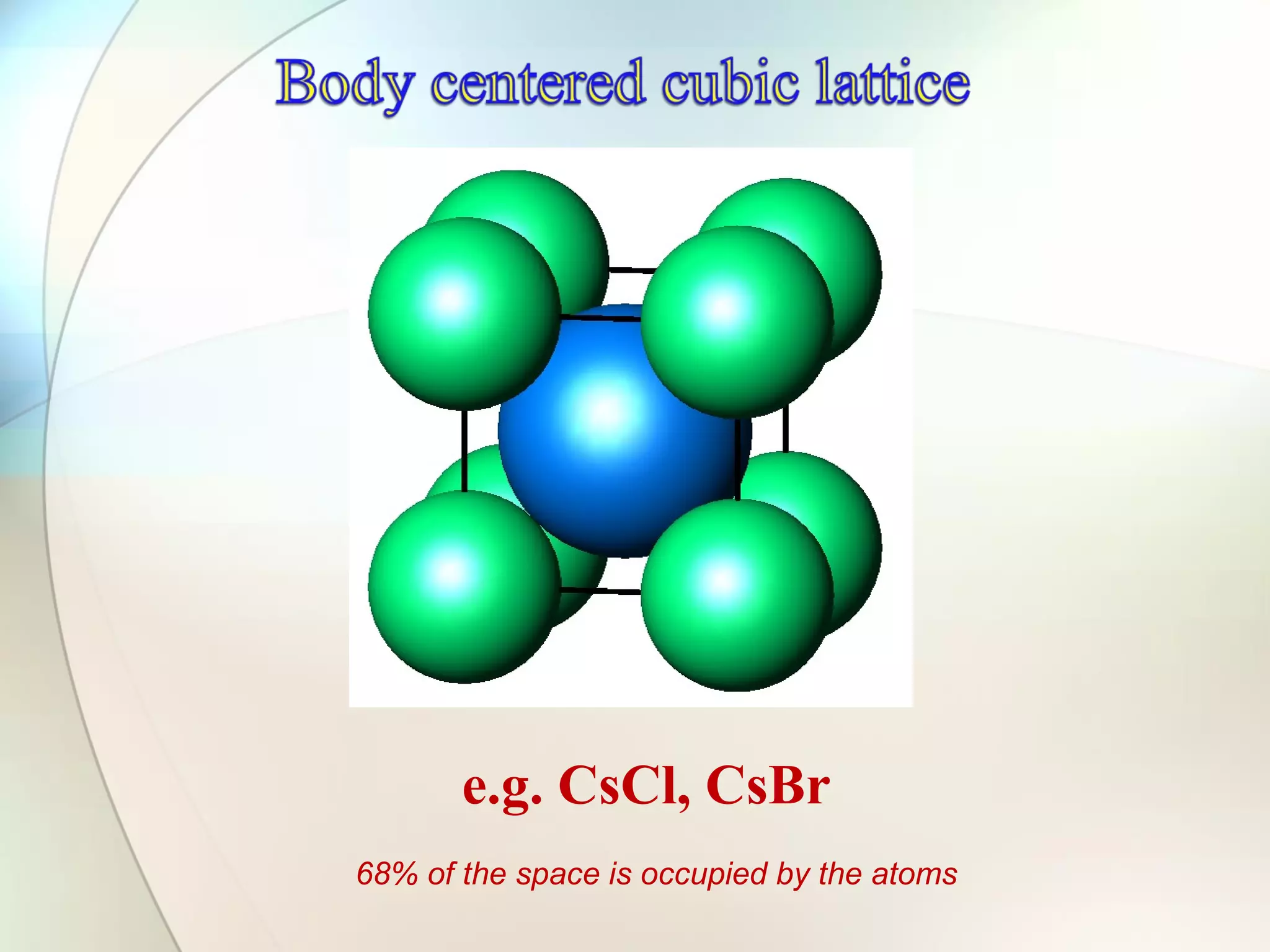
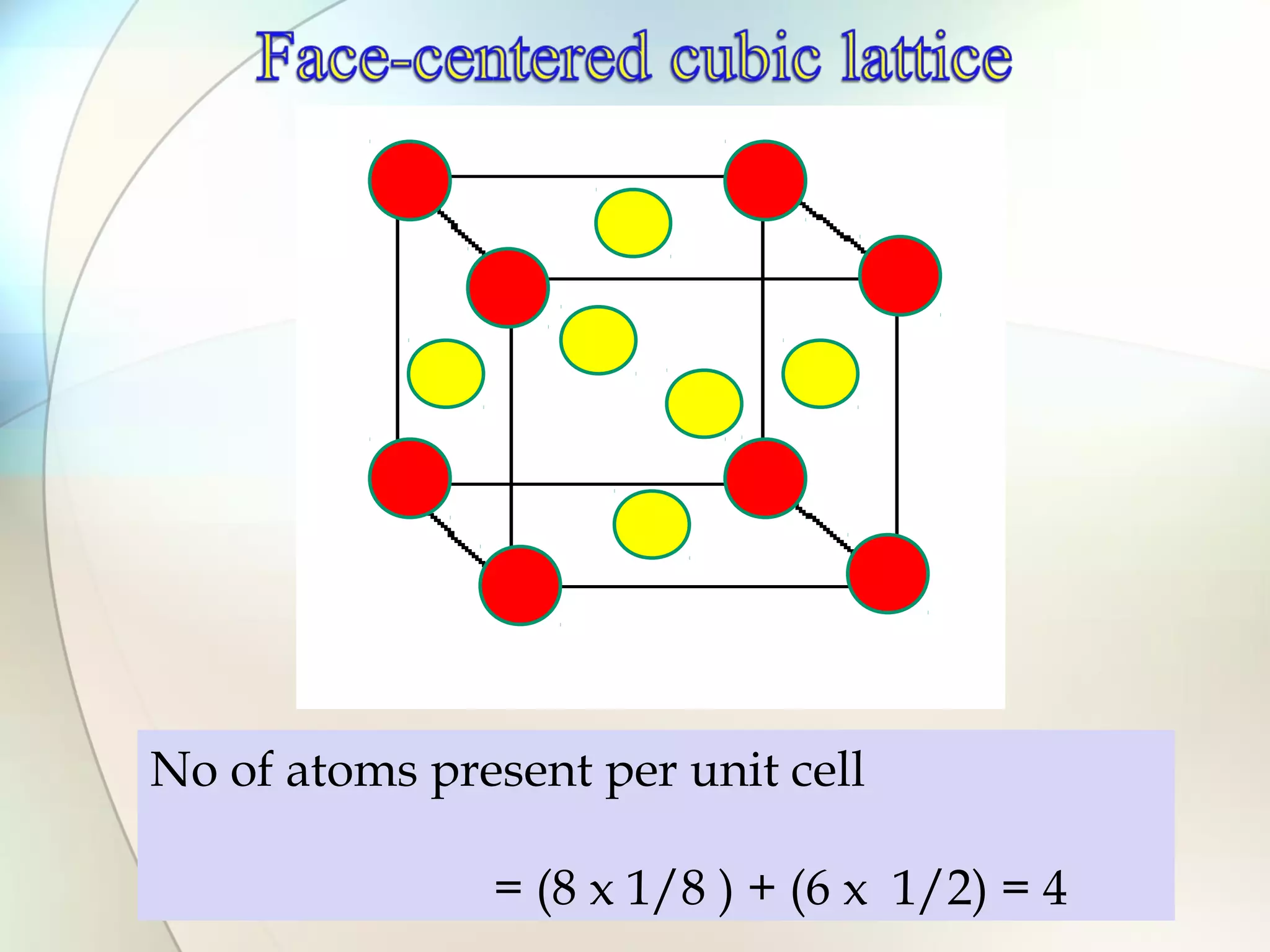
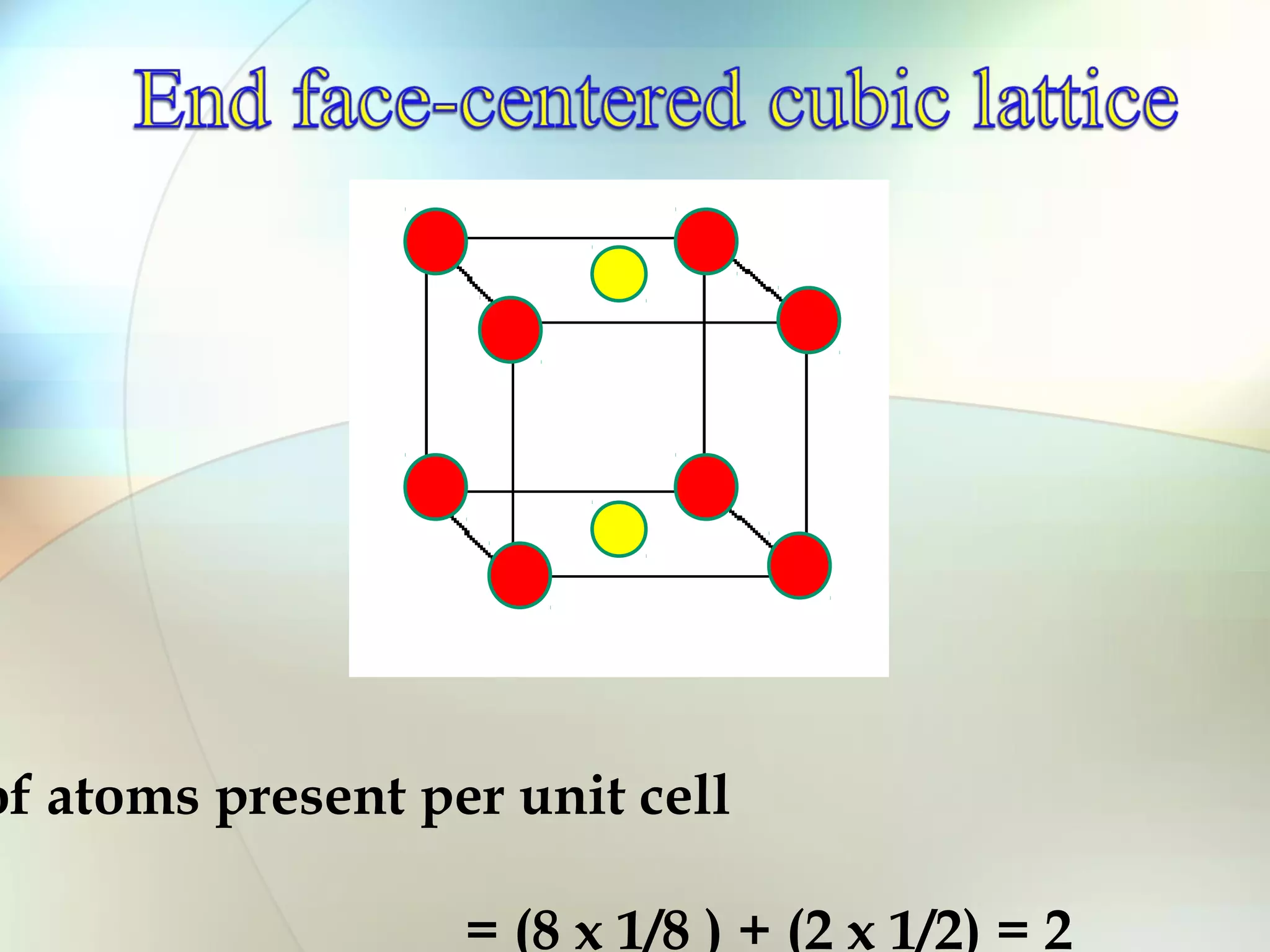
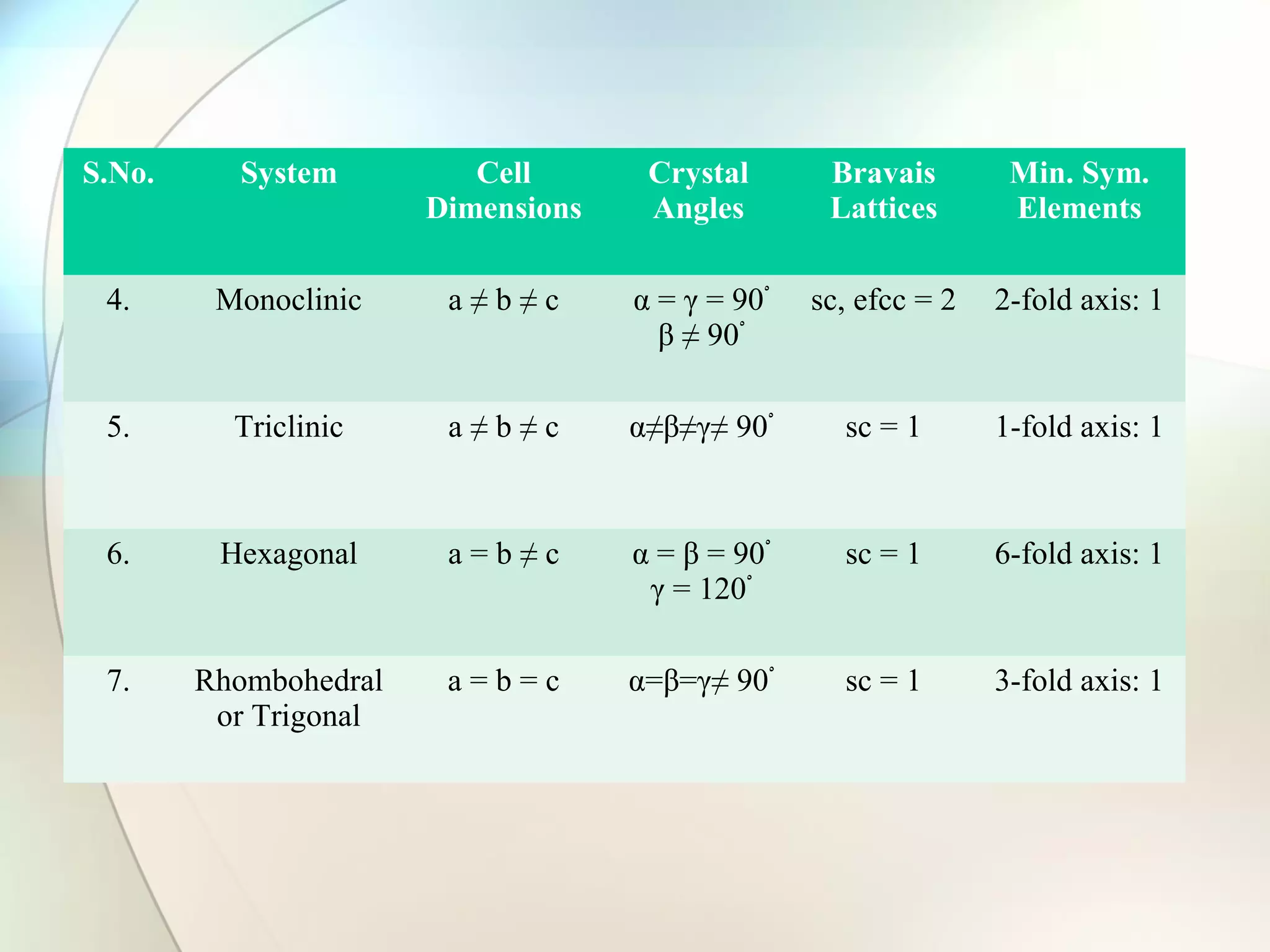
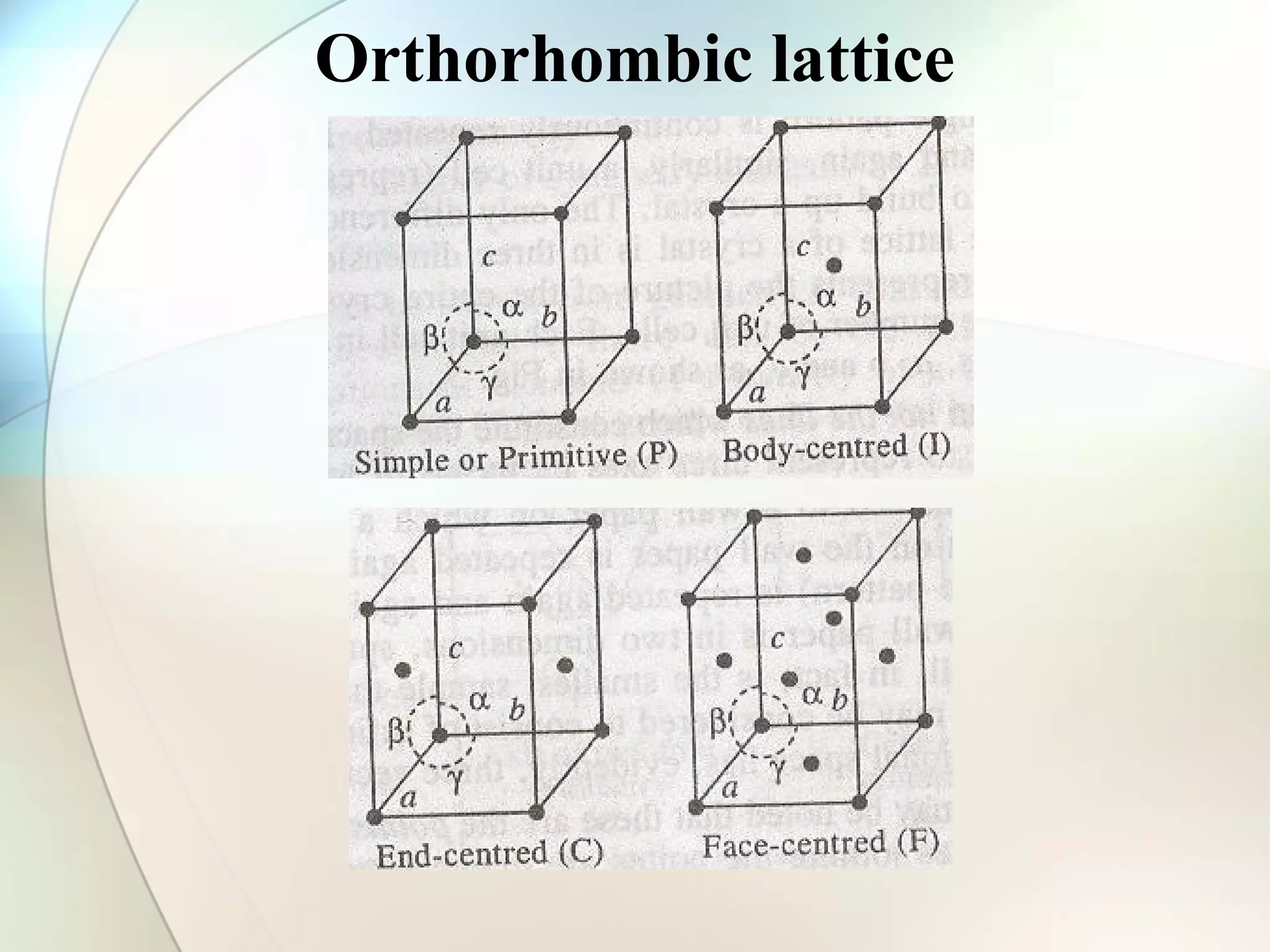
This document discusses solid state chemistry and provides information on various topics within the subject. It begins by defining the three states of matter and what distinguishes a solid. It then describes the two main types of solids - crystalline and amorphous - and provides details on their structures and properties. Various types of crystal structures are also outlined, including ionic, covalent, molecular and metallic crystals. The document concludes by discussing Bragg's equation and important solid materials like diamond, graphite and fullerenes.