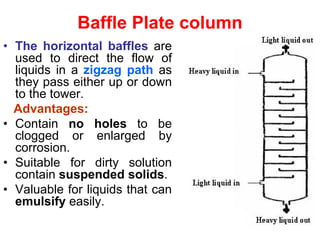
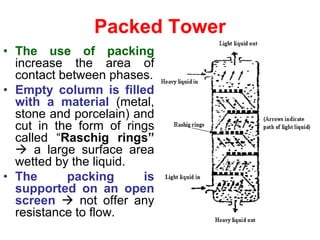
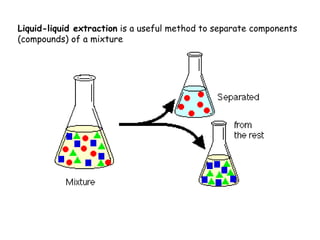
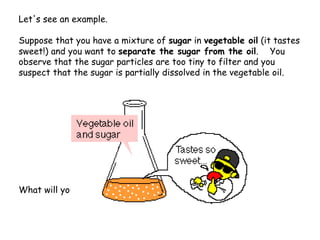
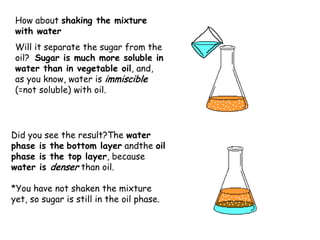
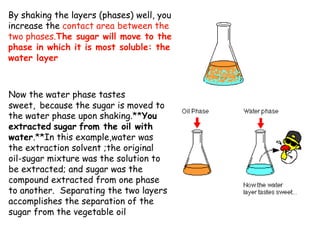
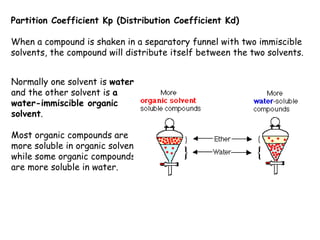
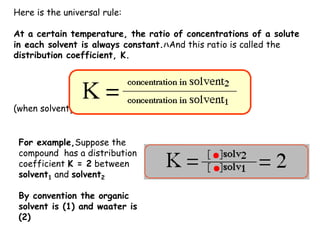
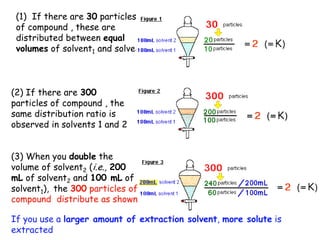
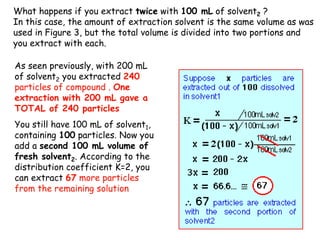
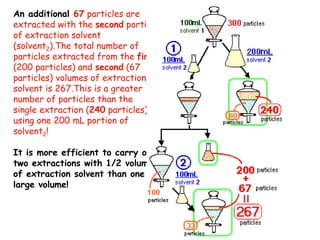
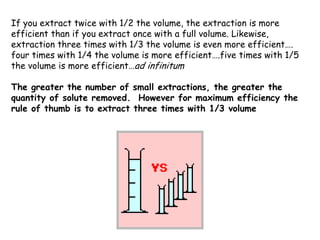
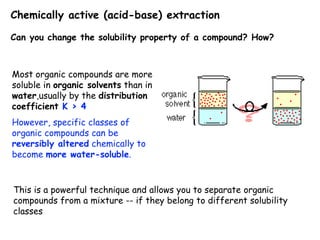
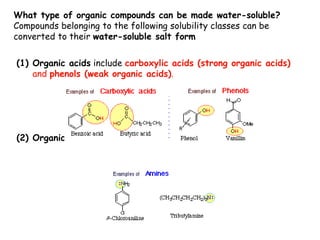
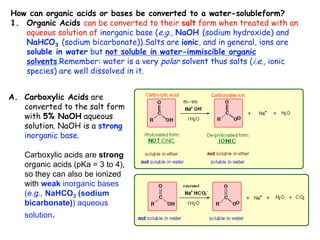
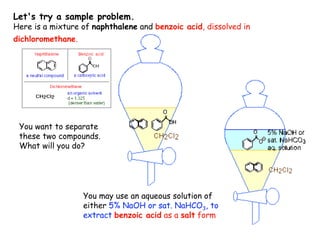
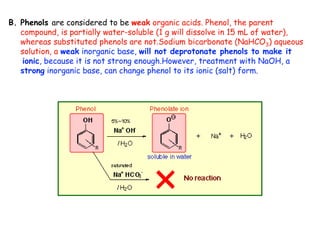
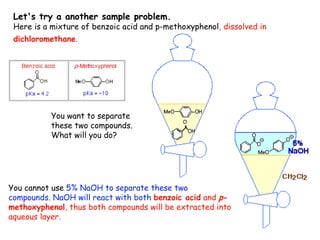
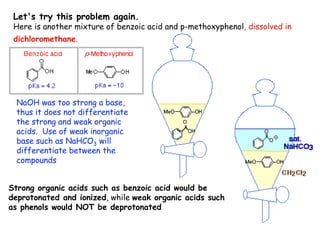
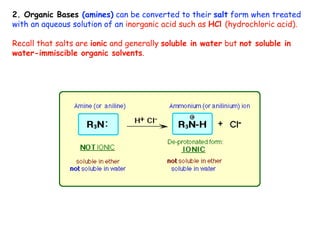
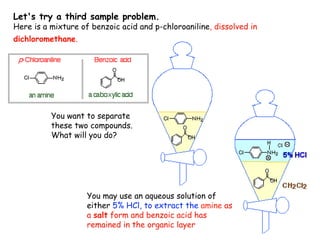
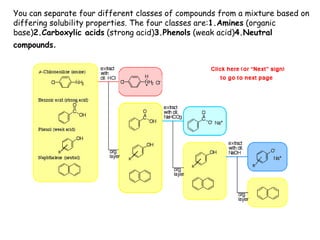
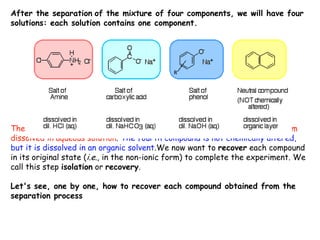
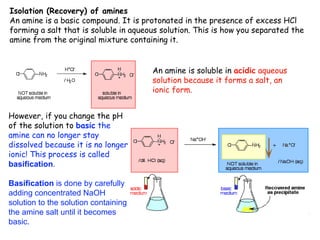
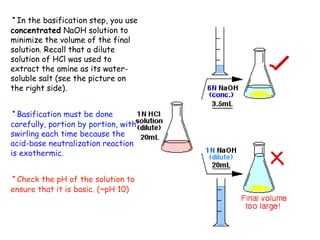
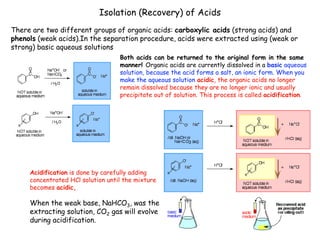
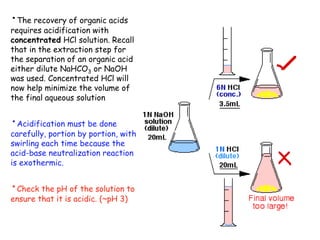
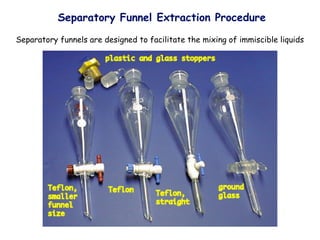
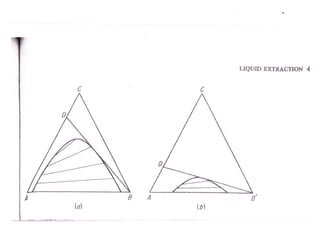
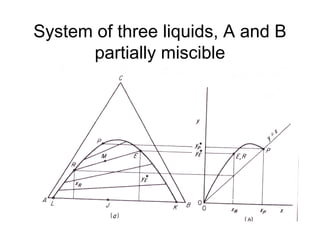
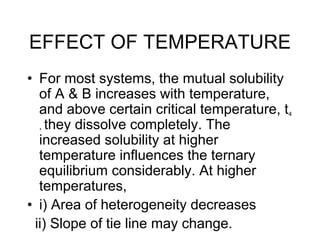
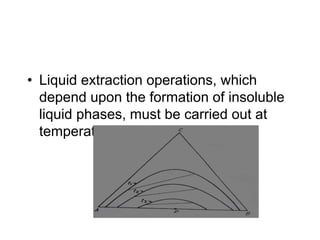
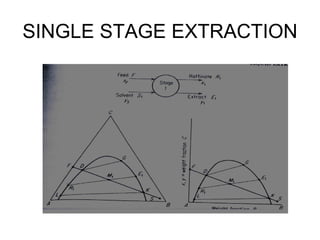
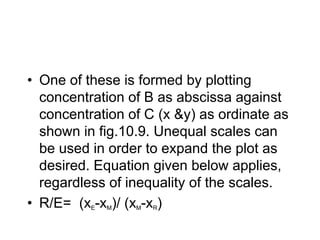
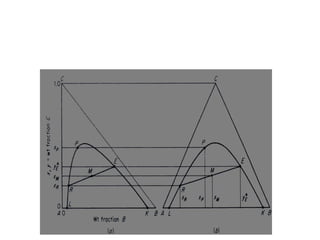
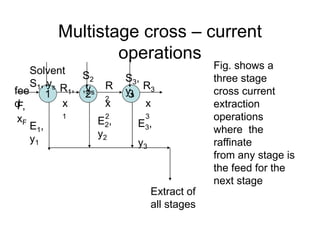
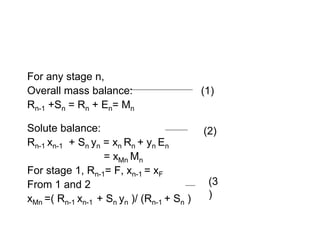
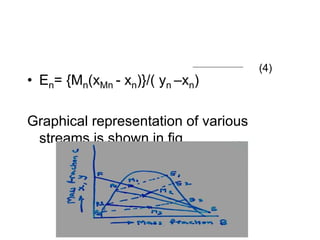
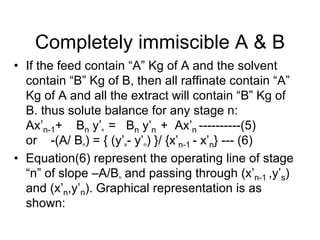
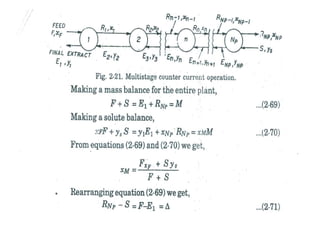
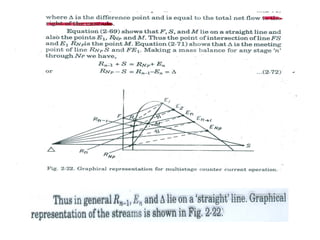
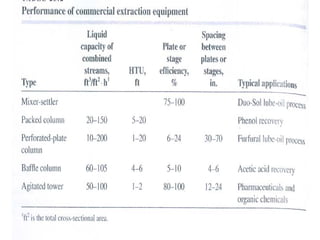
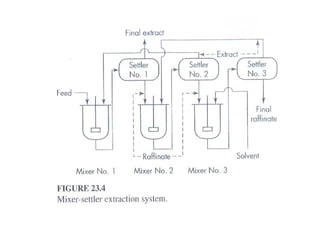
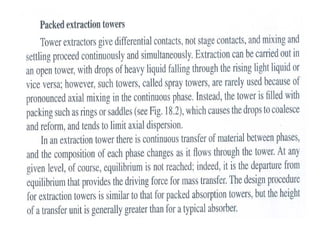
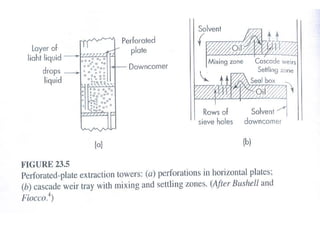
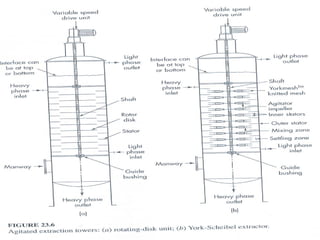
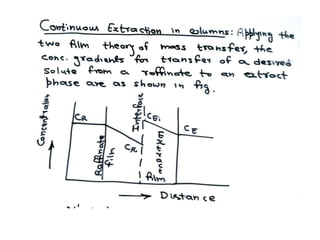
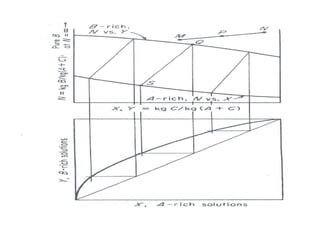
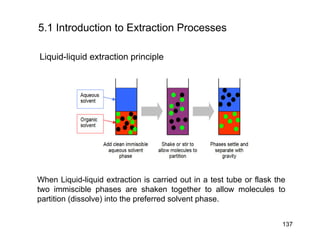
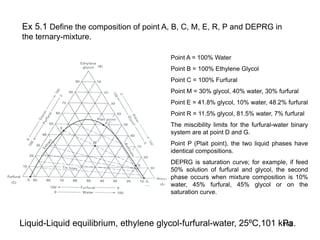
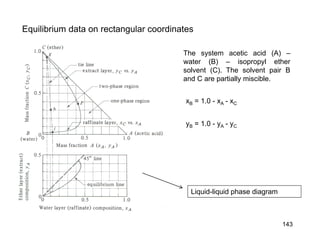
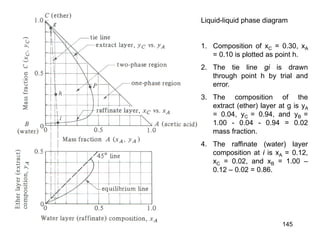
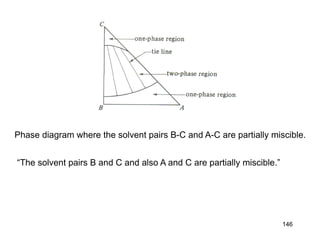
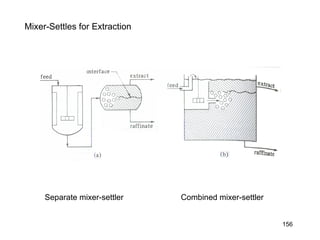
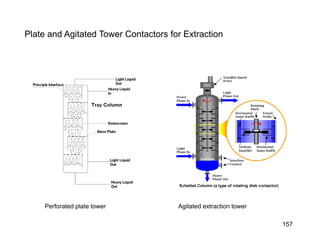
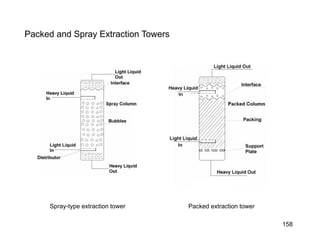
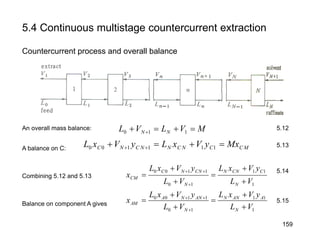
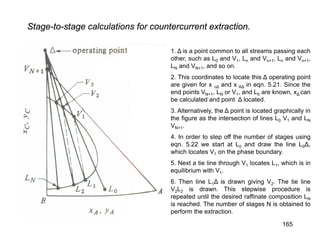
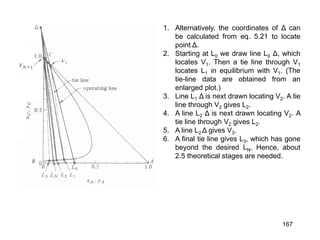
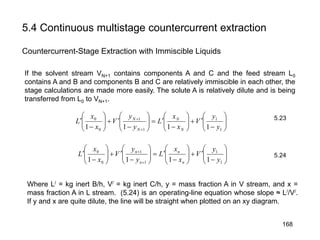
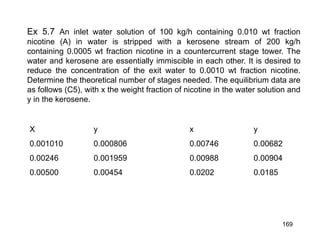
Extraction theory involves removing soluble materials from insolids using liquid solvents. Liquid-liquid extraction is a useful method to separate components of a mixture based on differences in solubility between solvents. For example, sugar can be extracted from vegetable oil by shaking the mixture with water, as sugar is more soluble in water than oil. The partition coefficient K quantifies differences in solubility, with some compounds made more water-soluble by conversion to ionic salt forms using acid or base treatment. This allows separation of organic acid/base mixtures based on differing solubility properties.










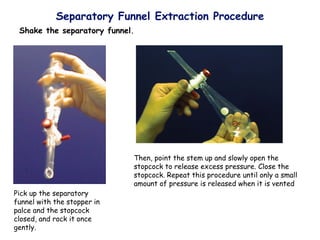
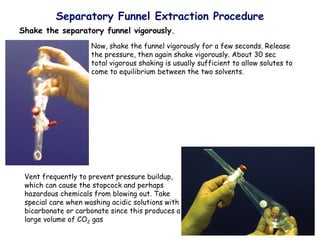



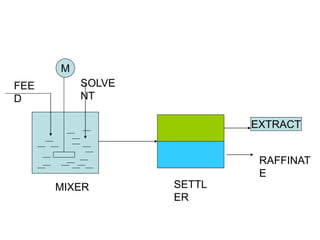




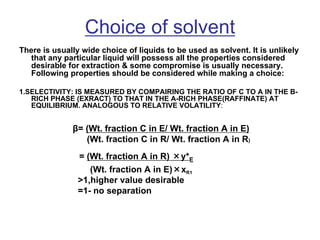










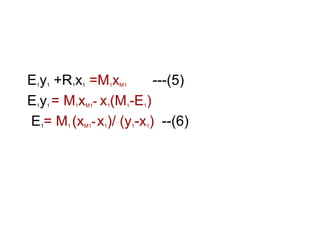




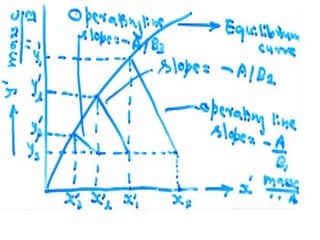
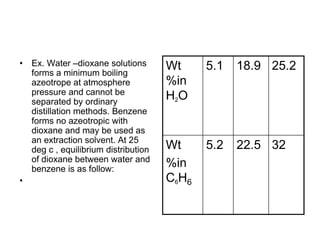

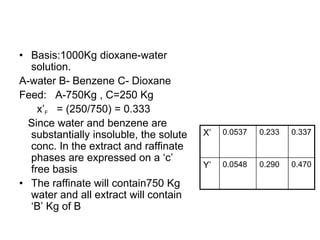

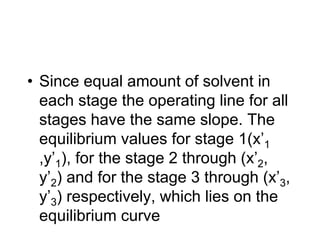

















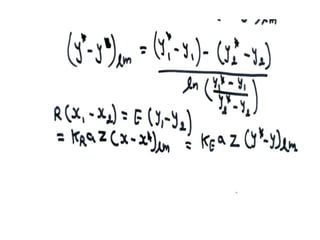






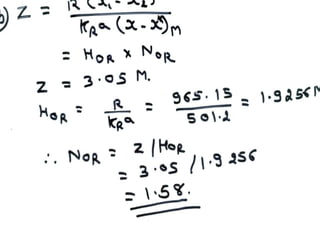












![ Define the following terms:
[Extraction, etc]
Respond to the following questions:
Give a detailed account of ………………
Explain in details the process of …………..
Describe in details with examples the…………
With examples, illustrate the pharmaceutical applications of ……………](https://image.slidesharecdn.com/15-extraction-200630111008/85/15-extraction-174-320.jpg)


