1) The document is a summary of Raoult's Law written by Muhammad Hamad Qureshi for the subject of Chemical Engineering Thermodynamics II.
2) Raoult's Law states that the vapor pressure of a solution is equal to the product of the vapor pressure of the pure solvent and the mole fraction of the solvent. The relative lowering of vapor pressure is directly proportional to the mole fraction of the solute.
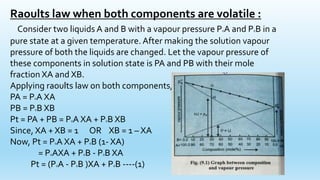
3) When both components in a solution are volatile, Raoult's Law can be expressed as an equation of a straight line. The vapor pressures of ideal solutions will fall on this straight line.