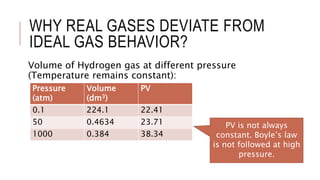
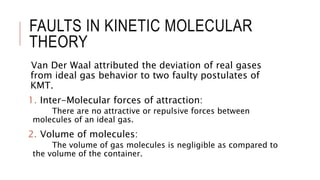
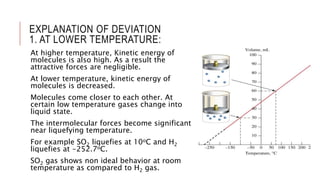
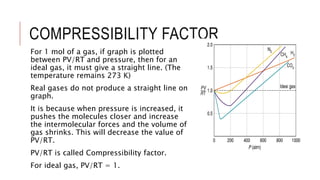
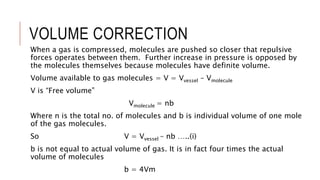
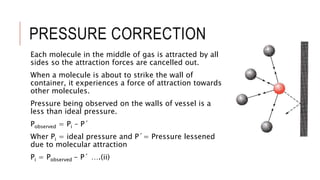
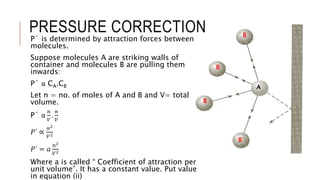
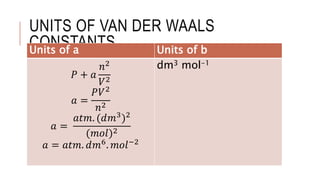
This document discusses the deviation of real gases from ideal gas behavior. It explains that real gases do not perfectly obey the ideal gas law PV=nRT under all conditions, especially at low temperatures and high pressures. This is because the kinetic molecular theory, which the ideal gas law is based on, does not account for intermolecular forces and the finite volume of gas molecules. The document then introduces the Van Der Waals equation, which includes correction factors for volume and pressure and provides a better model for the behavior of real gases.