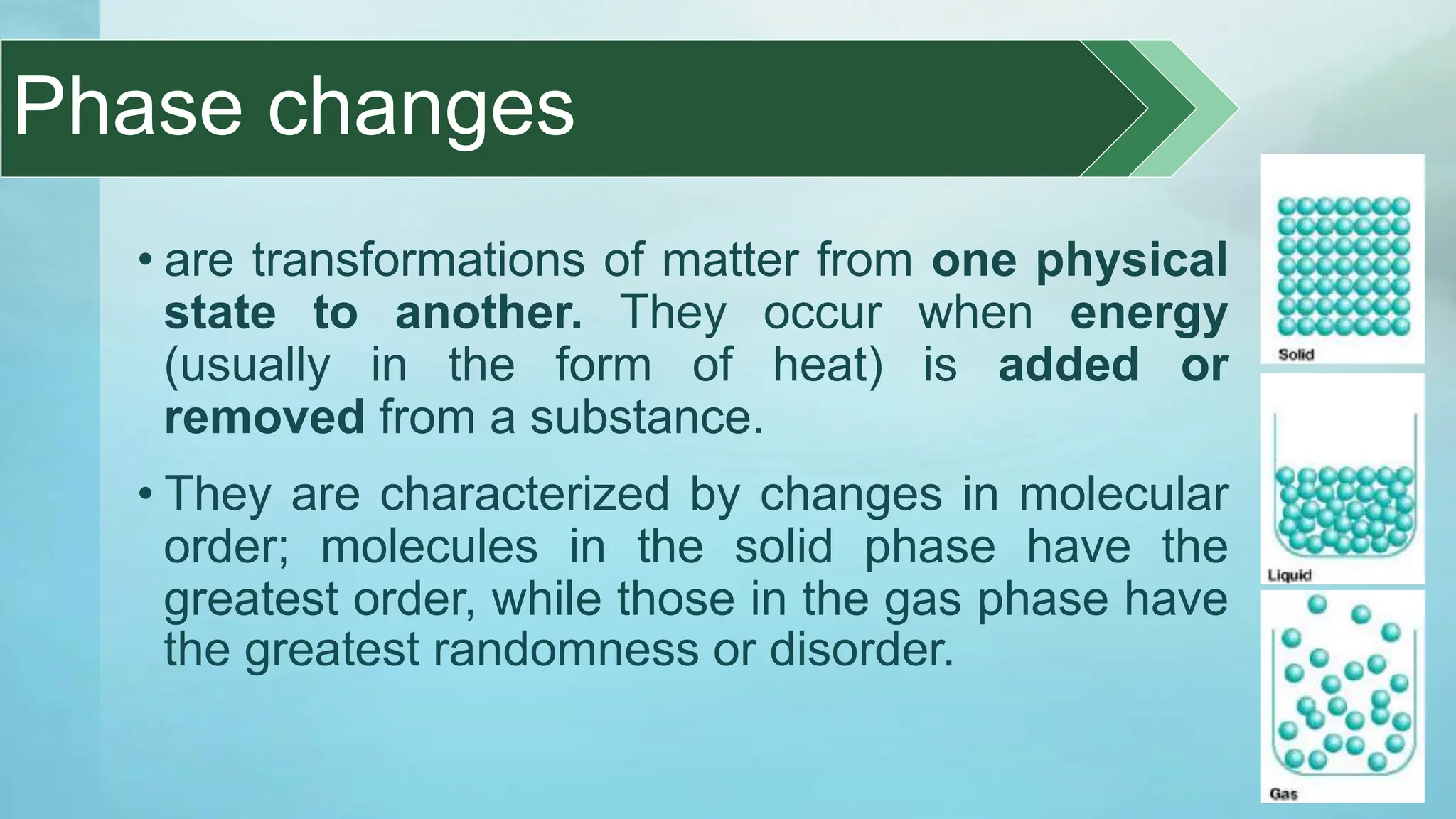
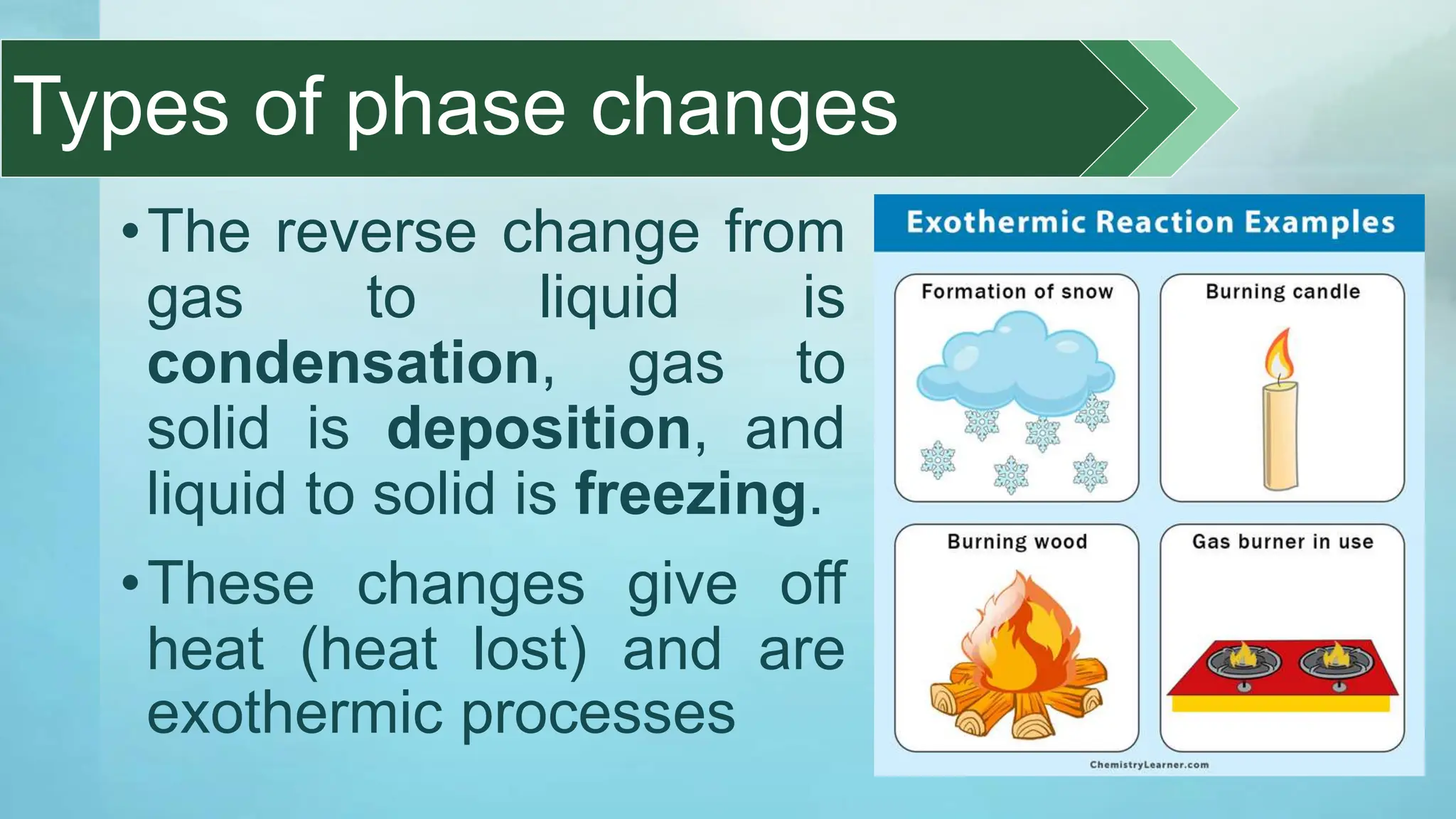
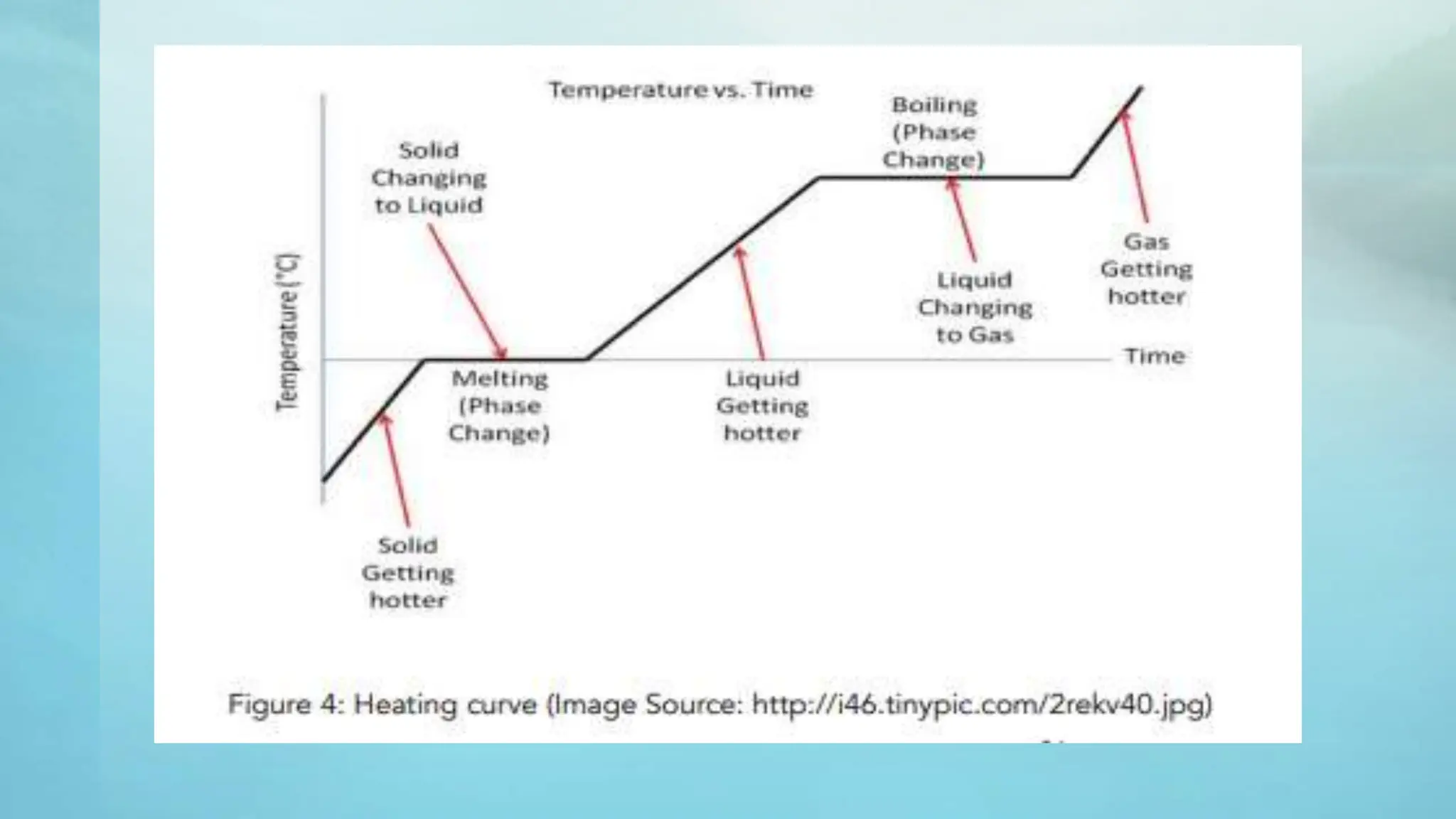
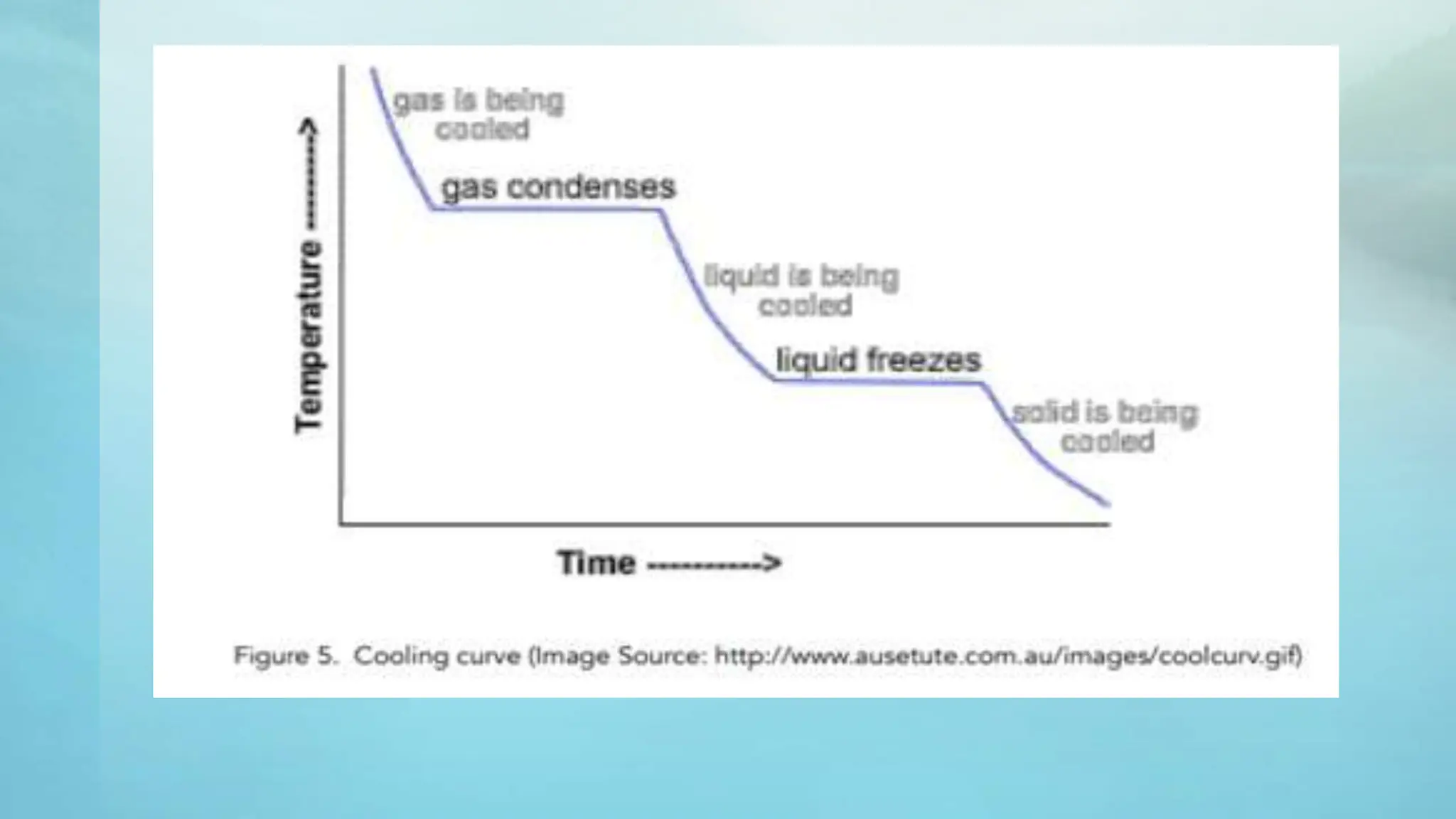
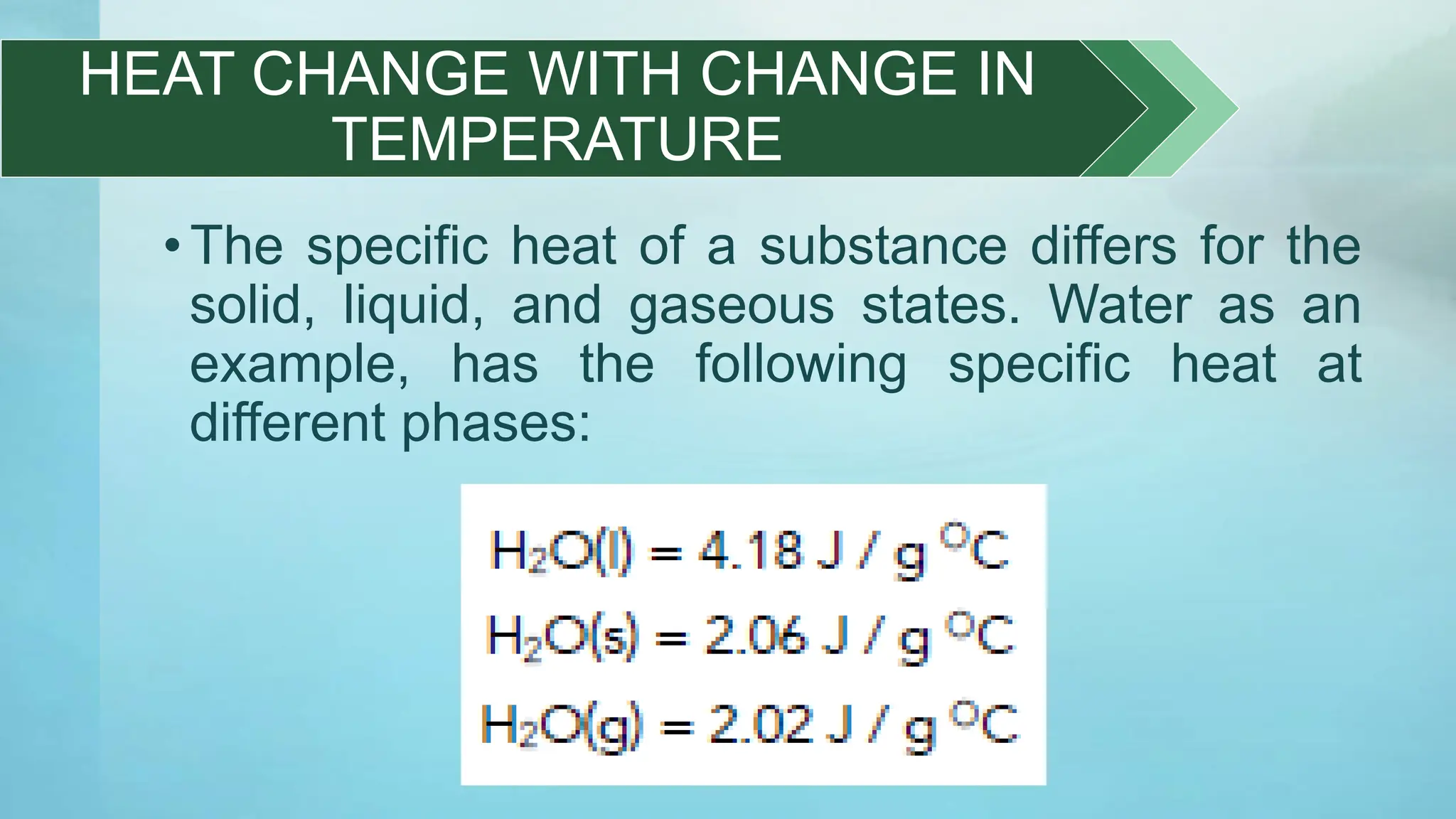
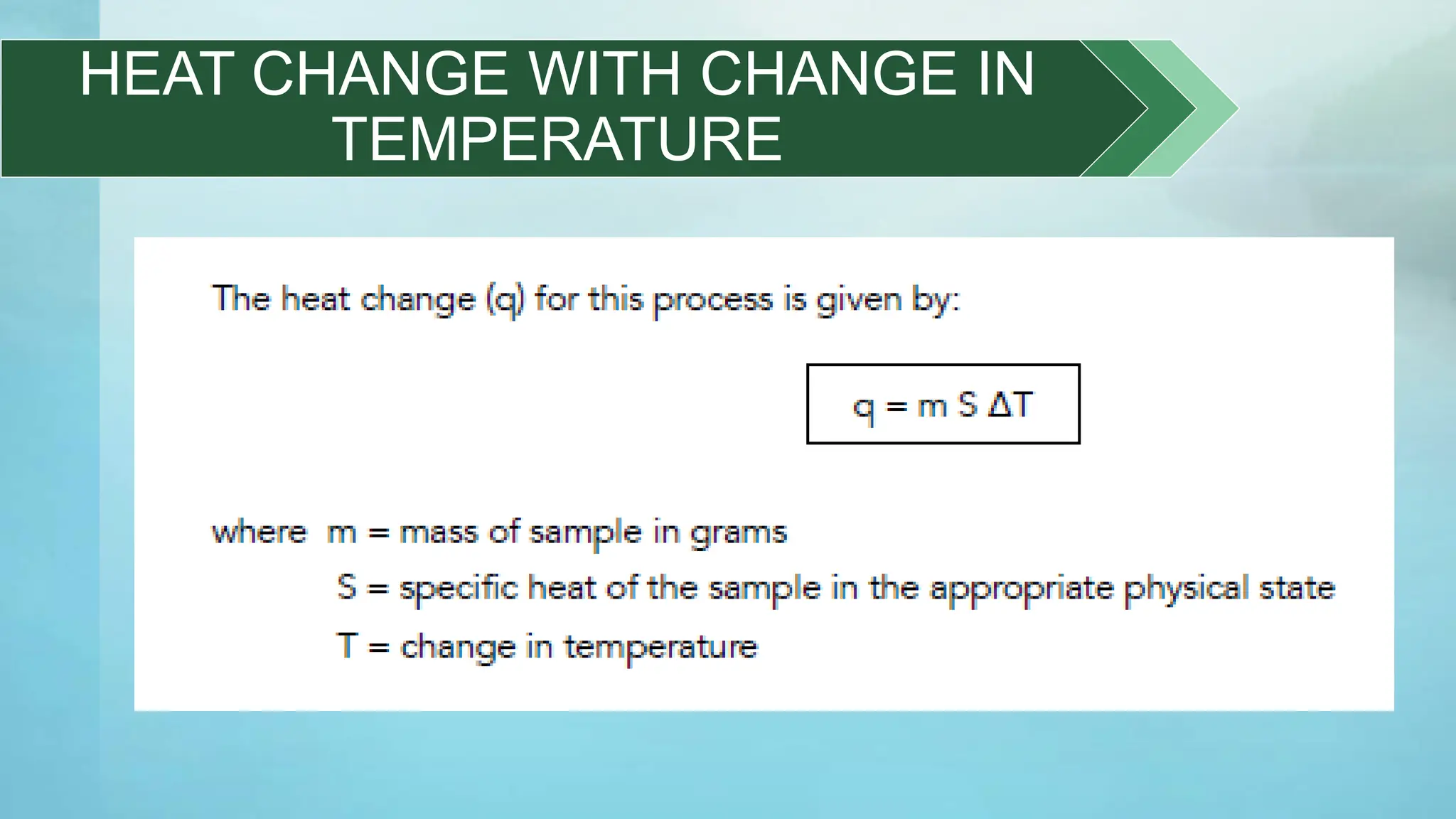
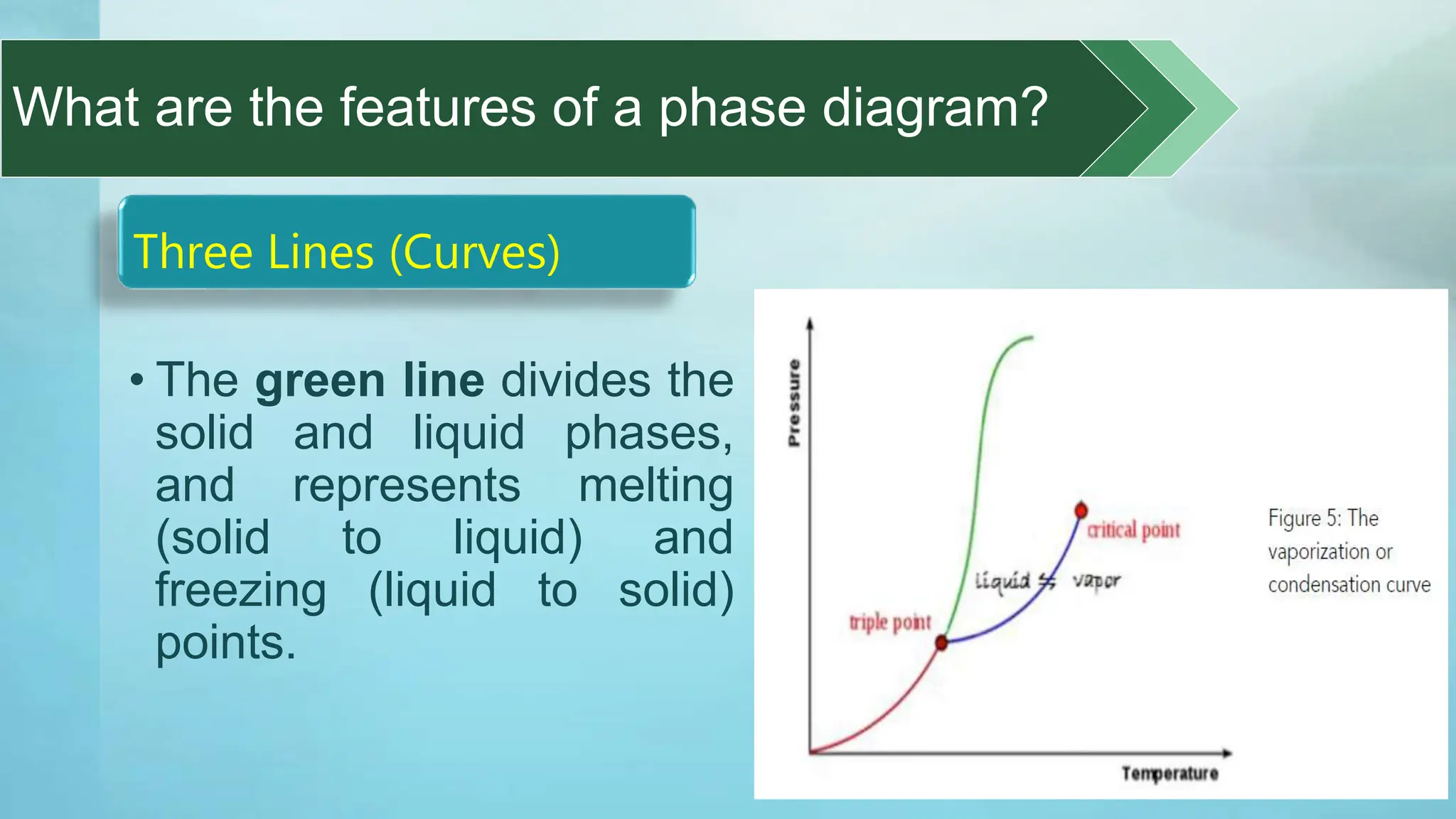
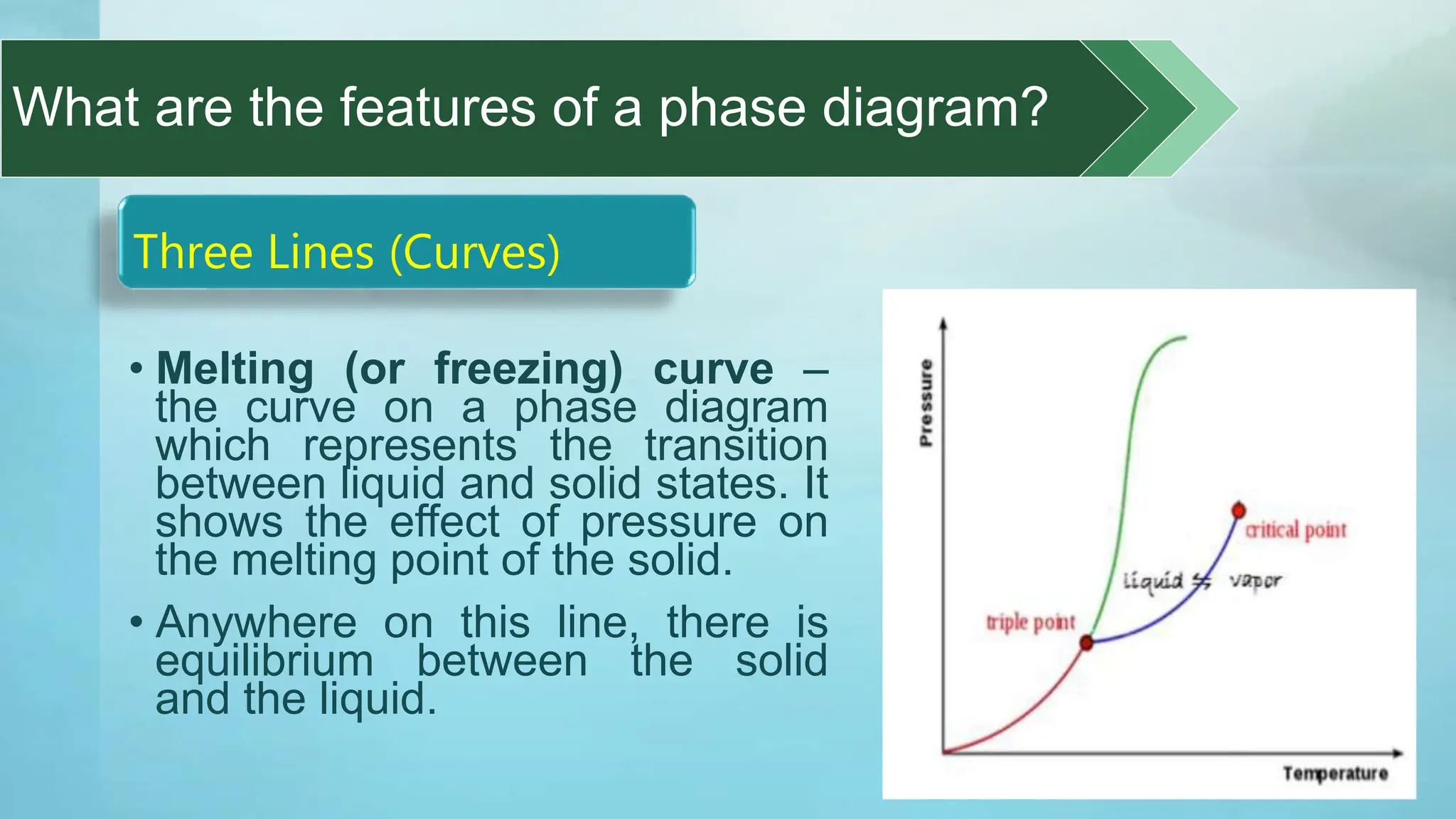
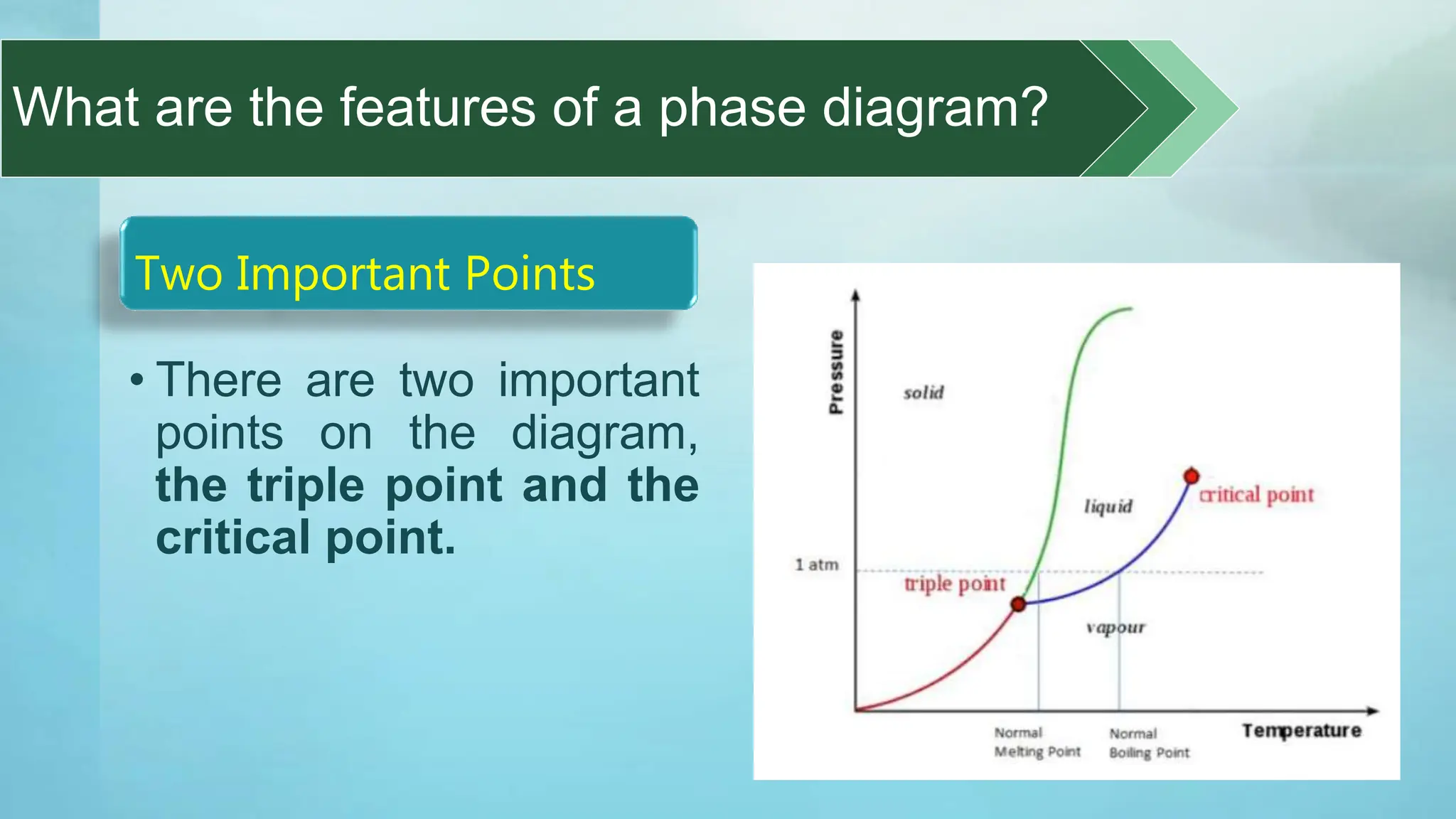
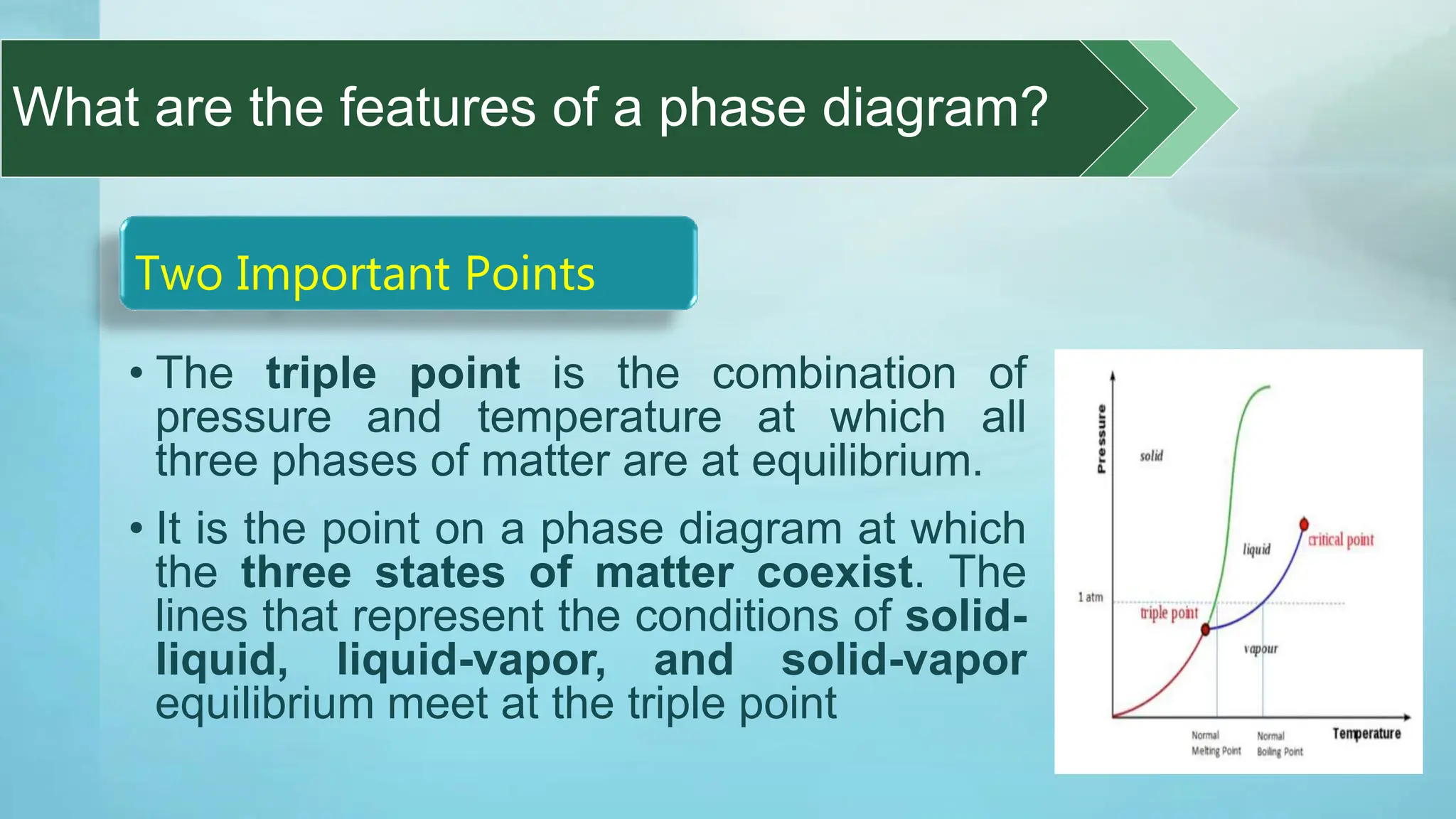
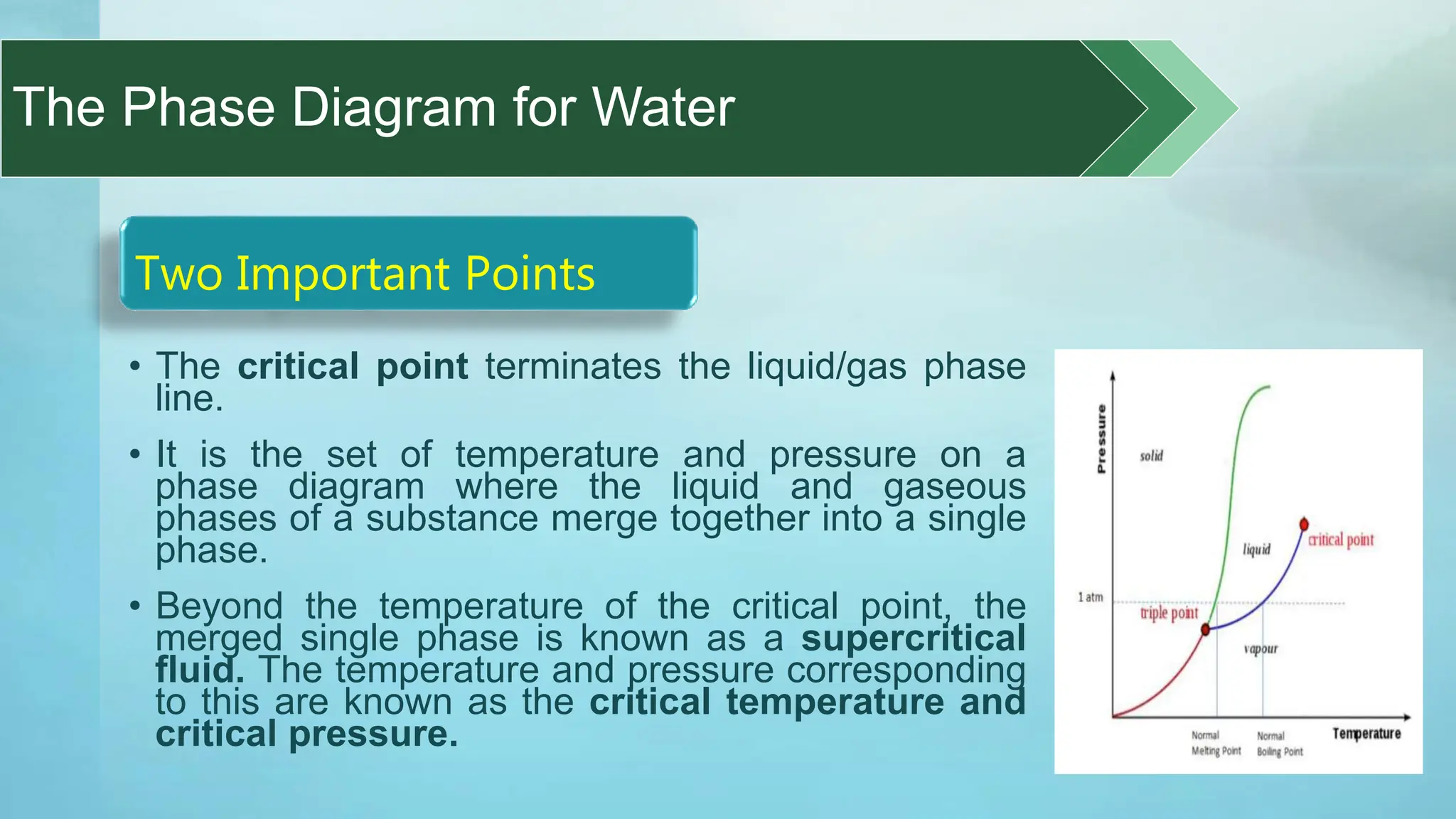
The document provides an educational overview of phase changes, defining key concepts and processes such as melting, freezing, vaporization, and condensation. It also discusses the significance of energy changes during phase transitions and introduces phase diagrams, which graphically represent the states of matter under various temperature and pressure conditions. The document outlines learning objectives related to explaining, interpreting, and analyzing these concepts in the context of different substances.