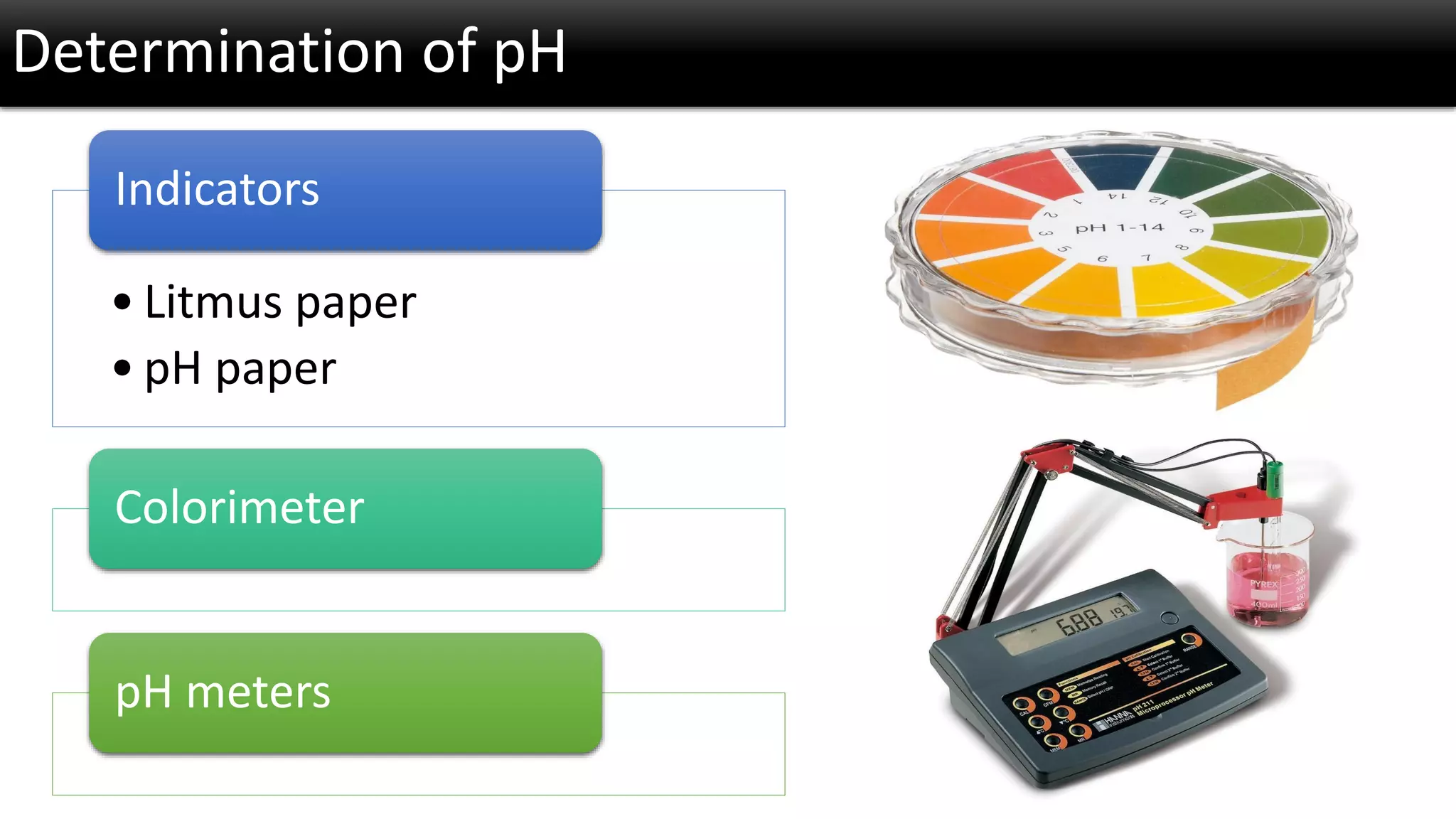
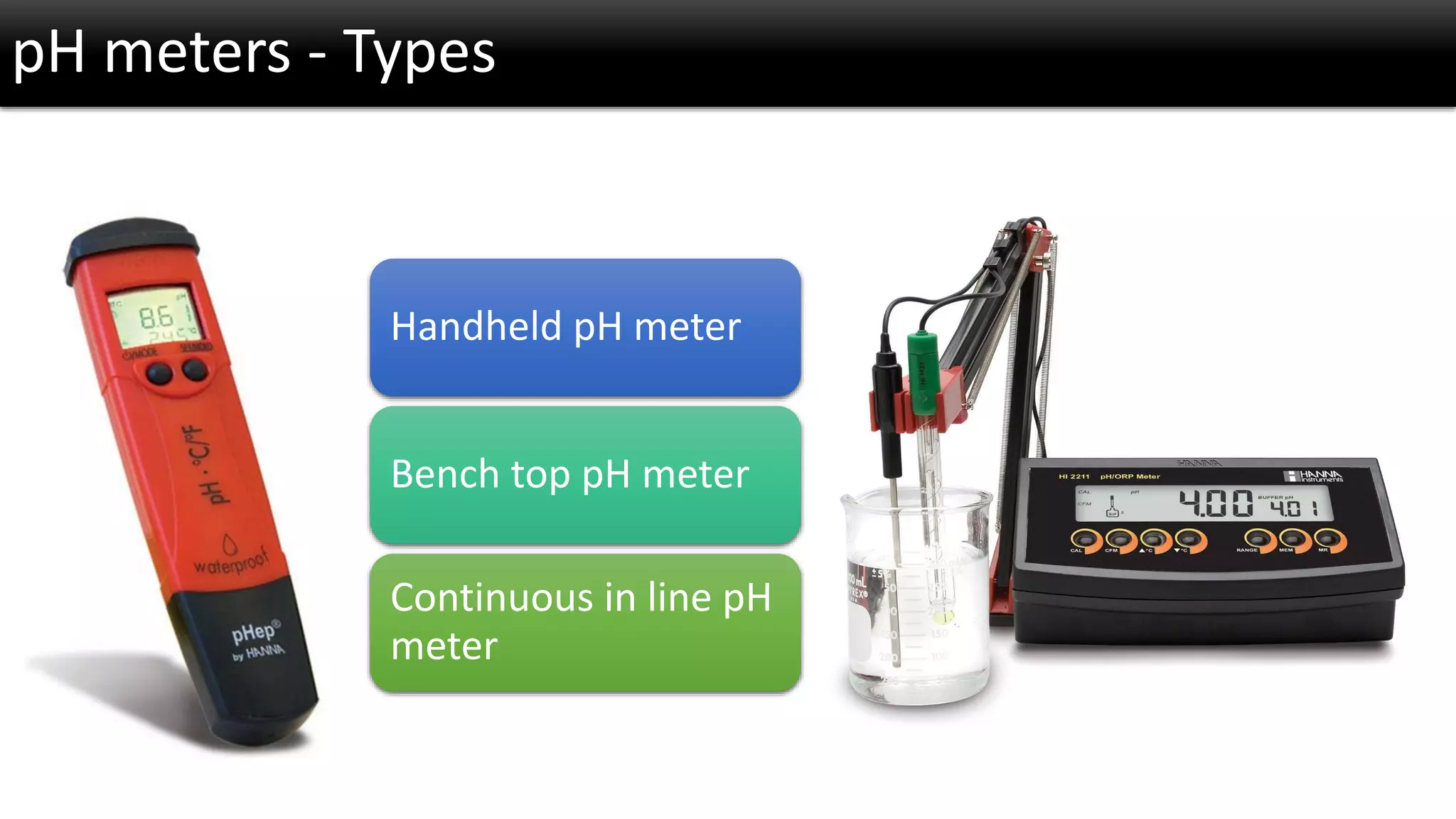
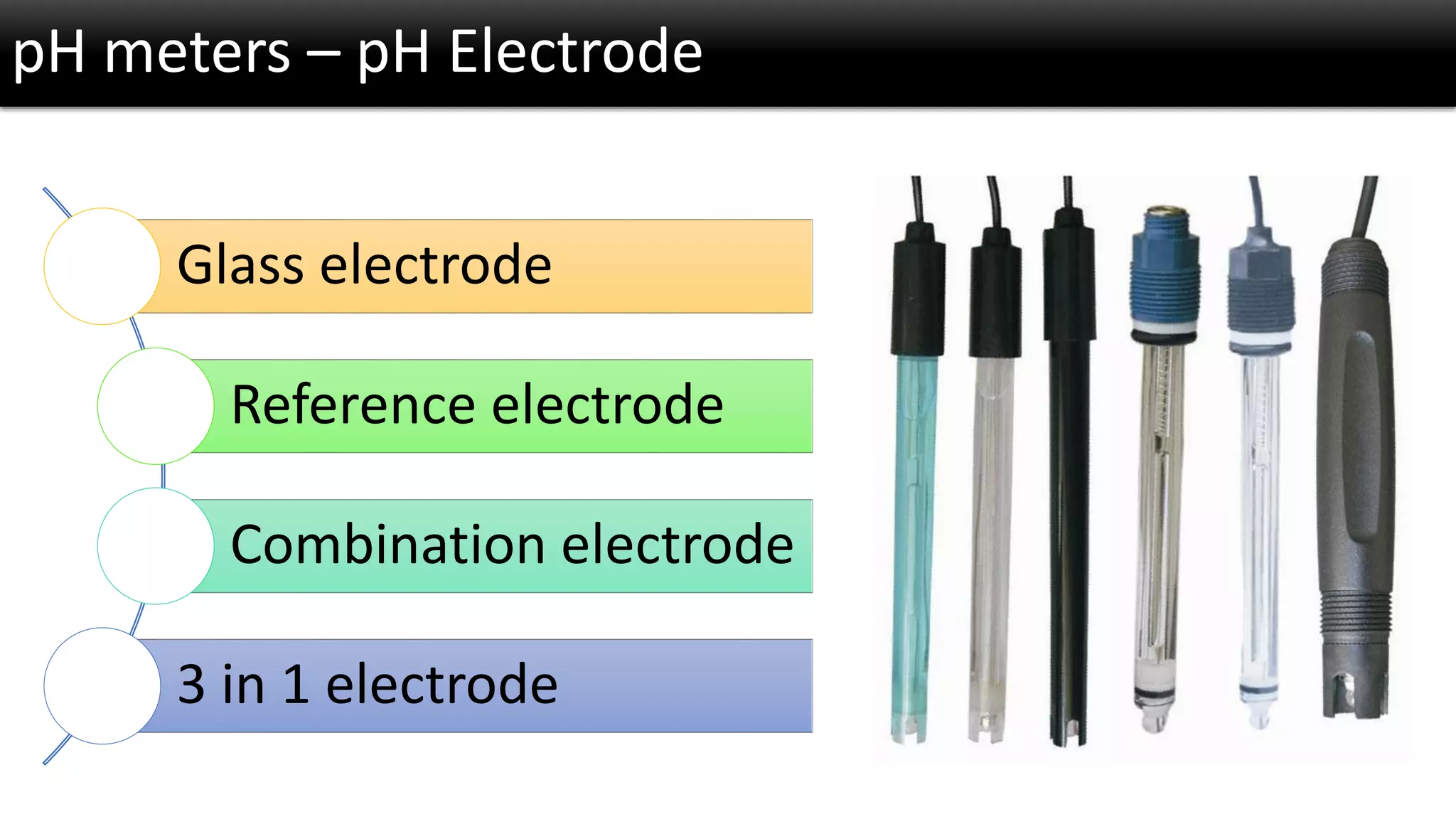
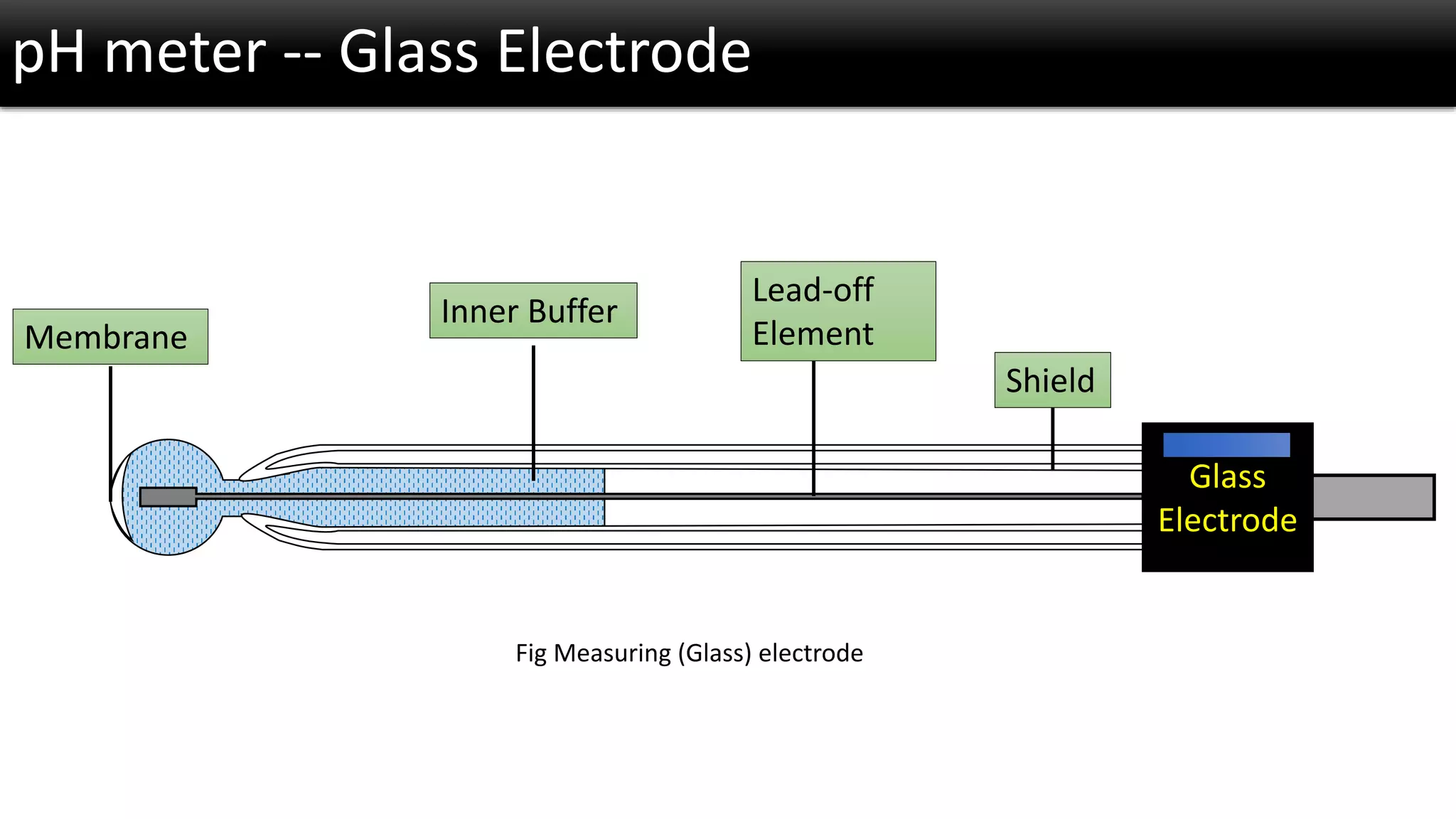
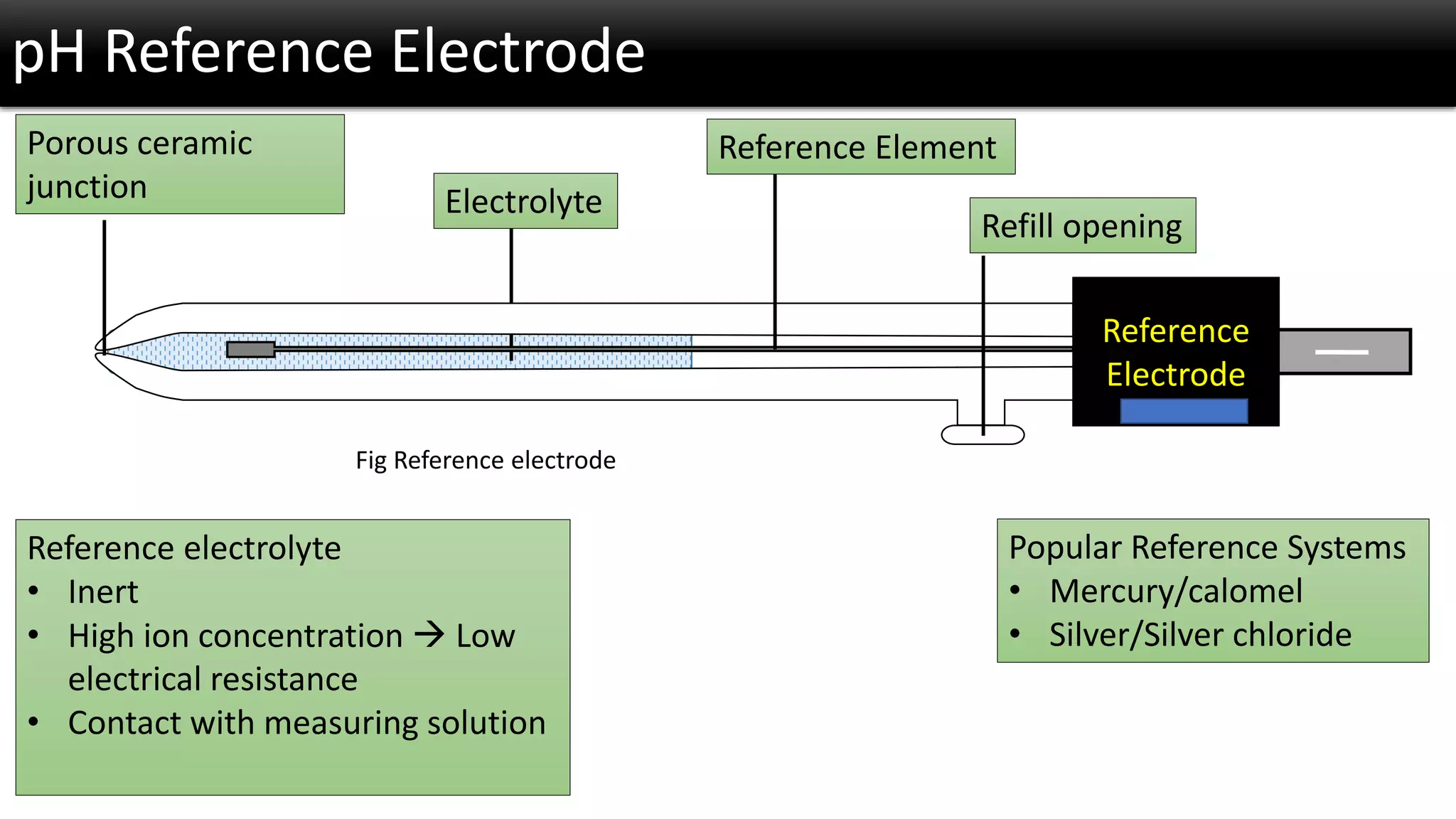
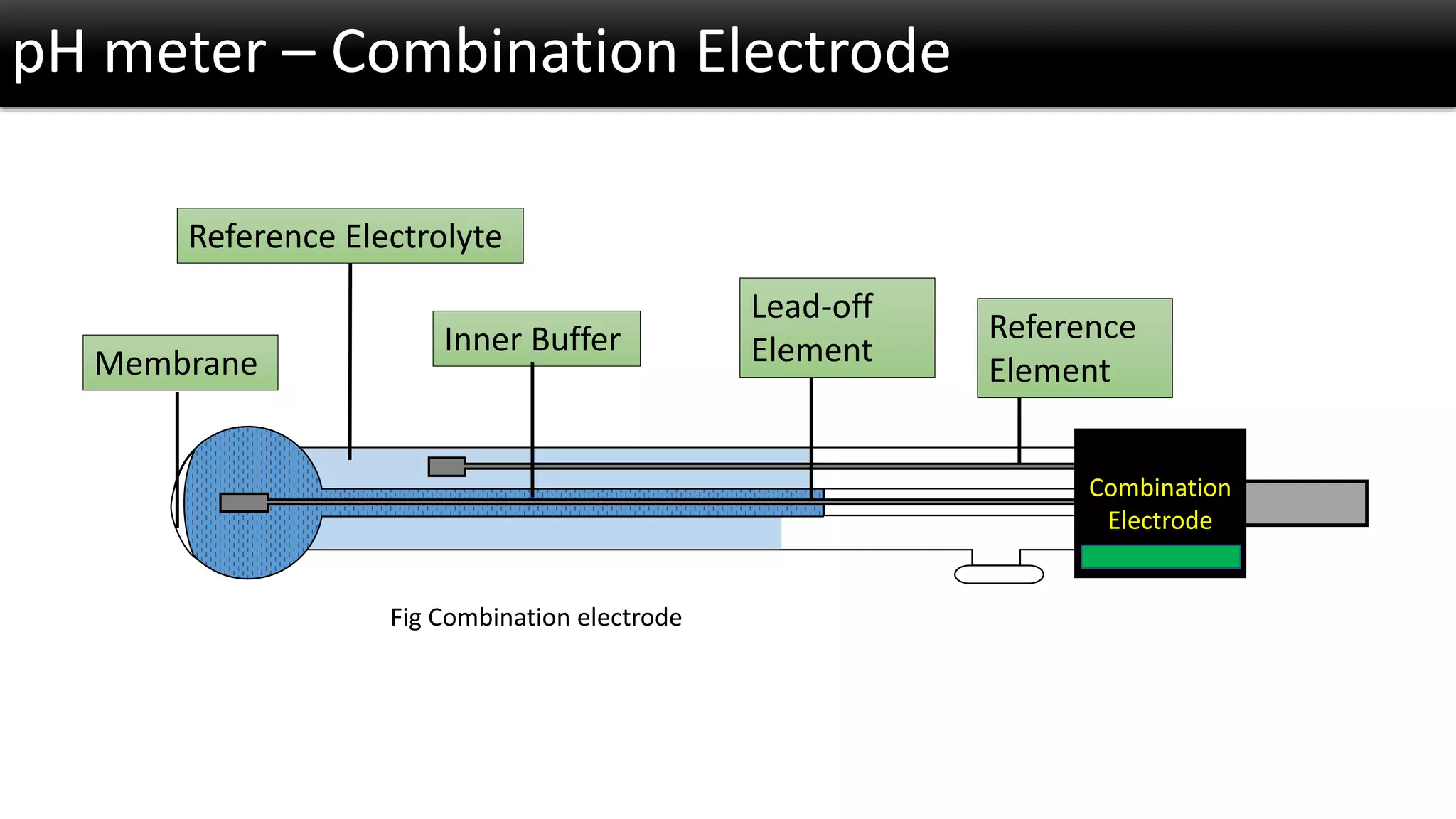
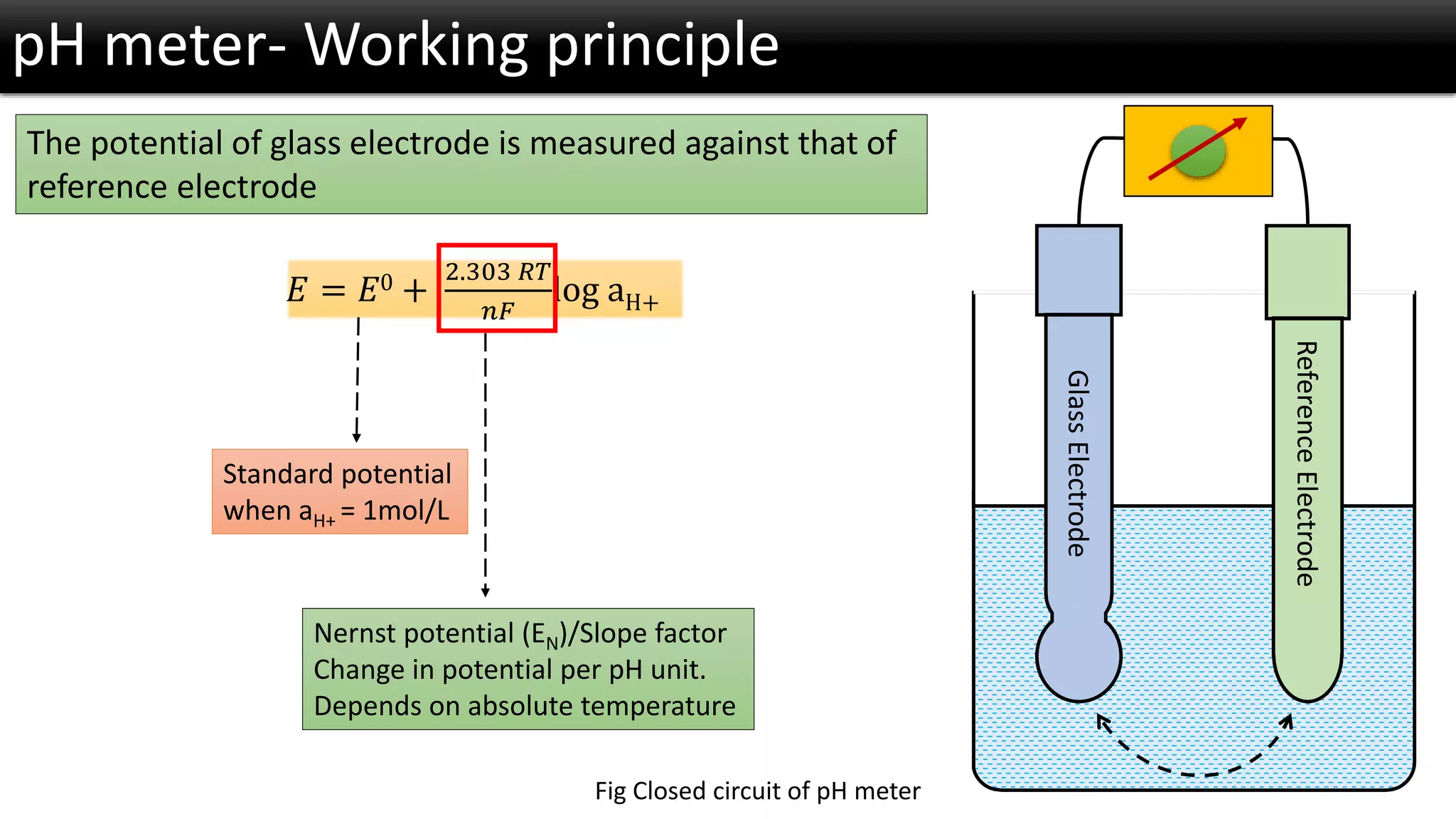
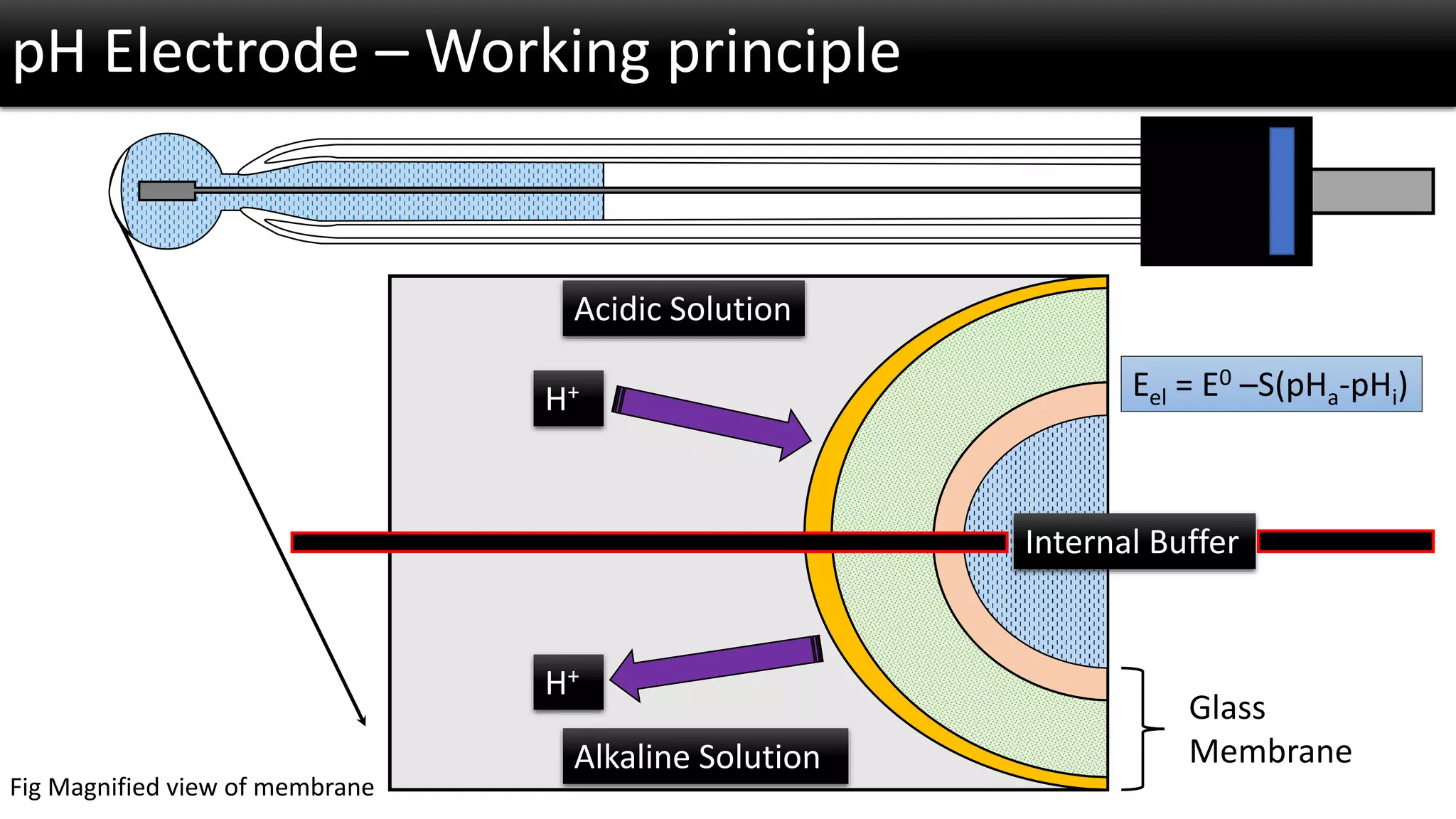
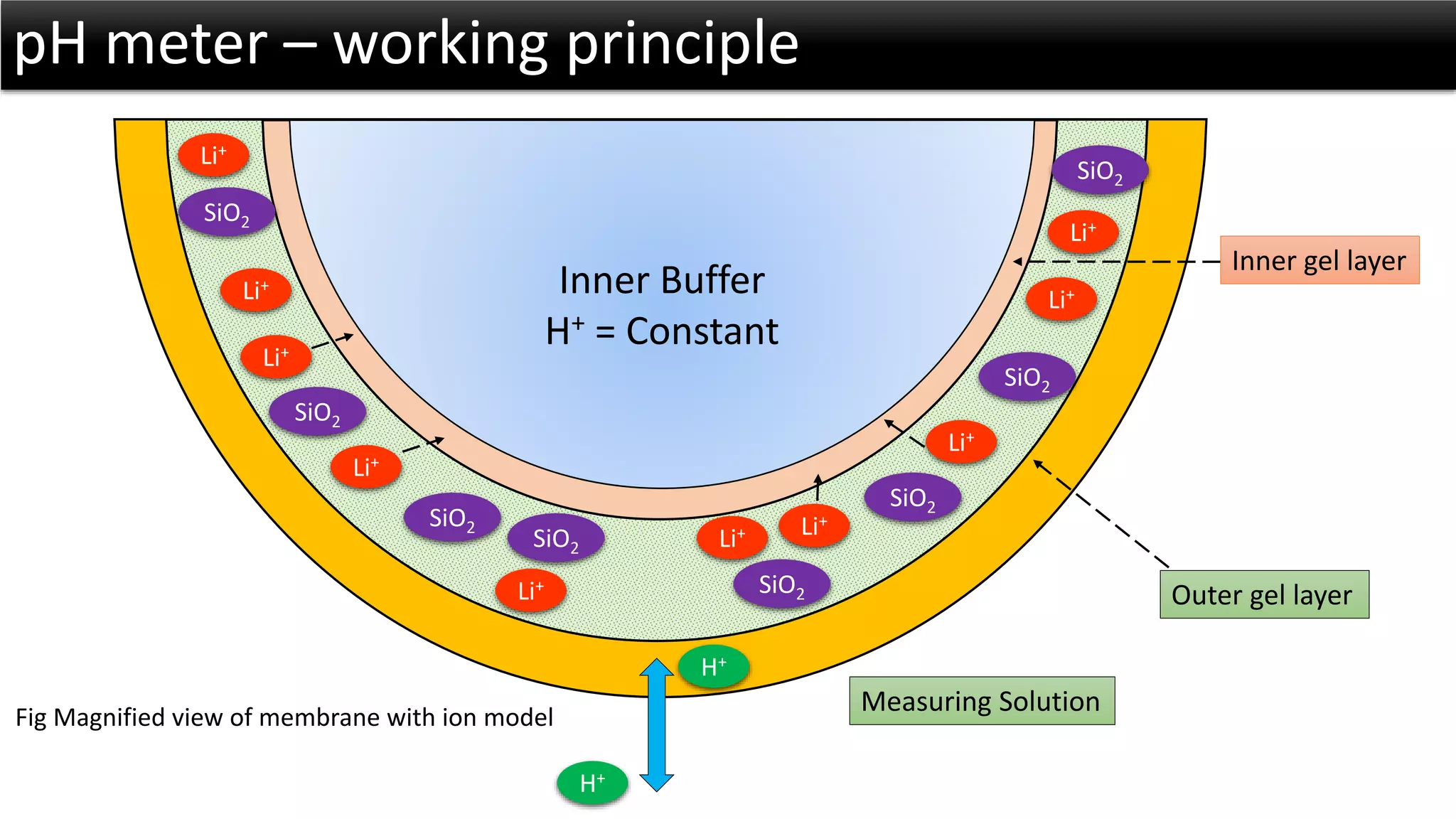
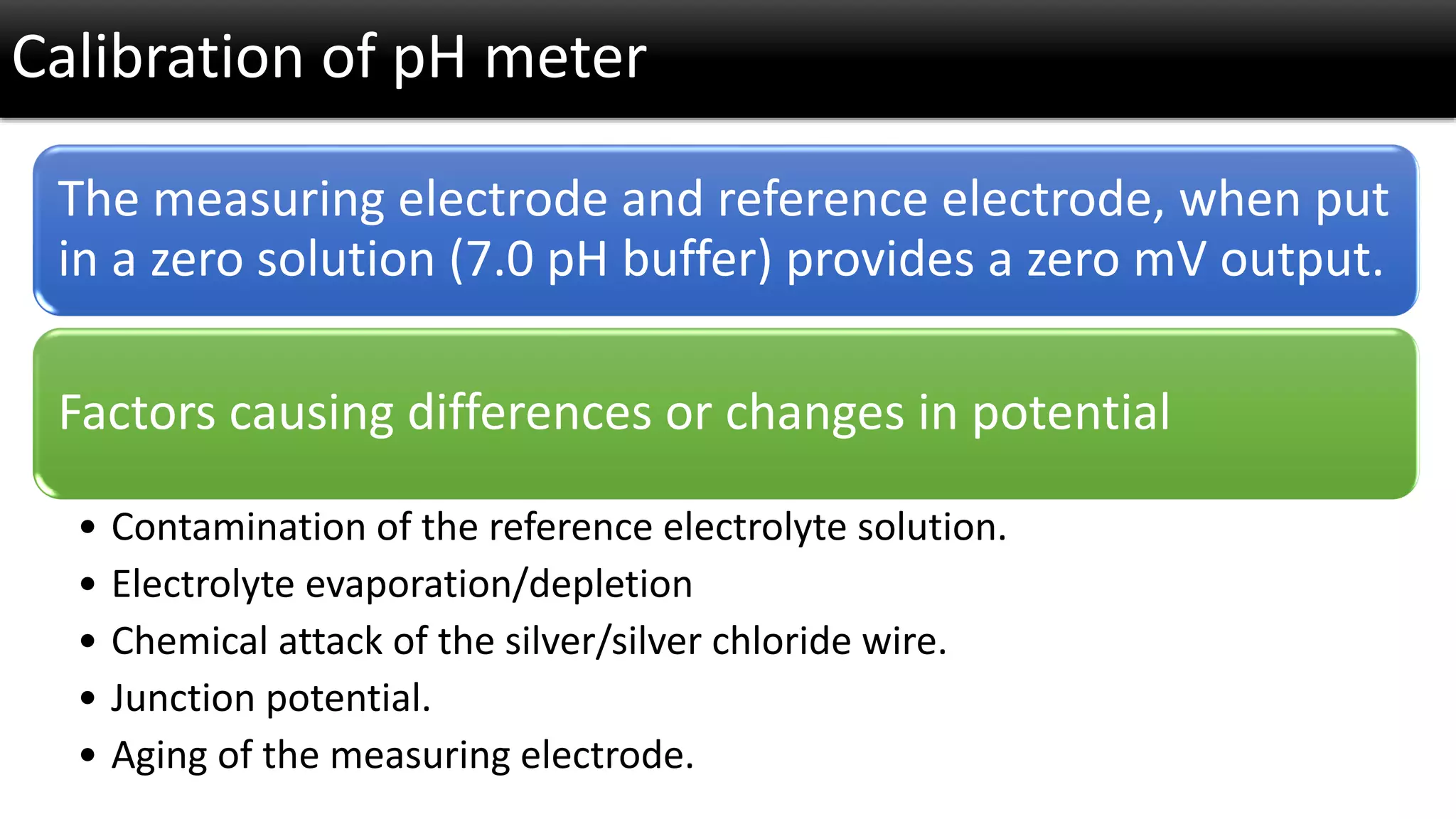
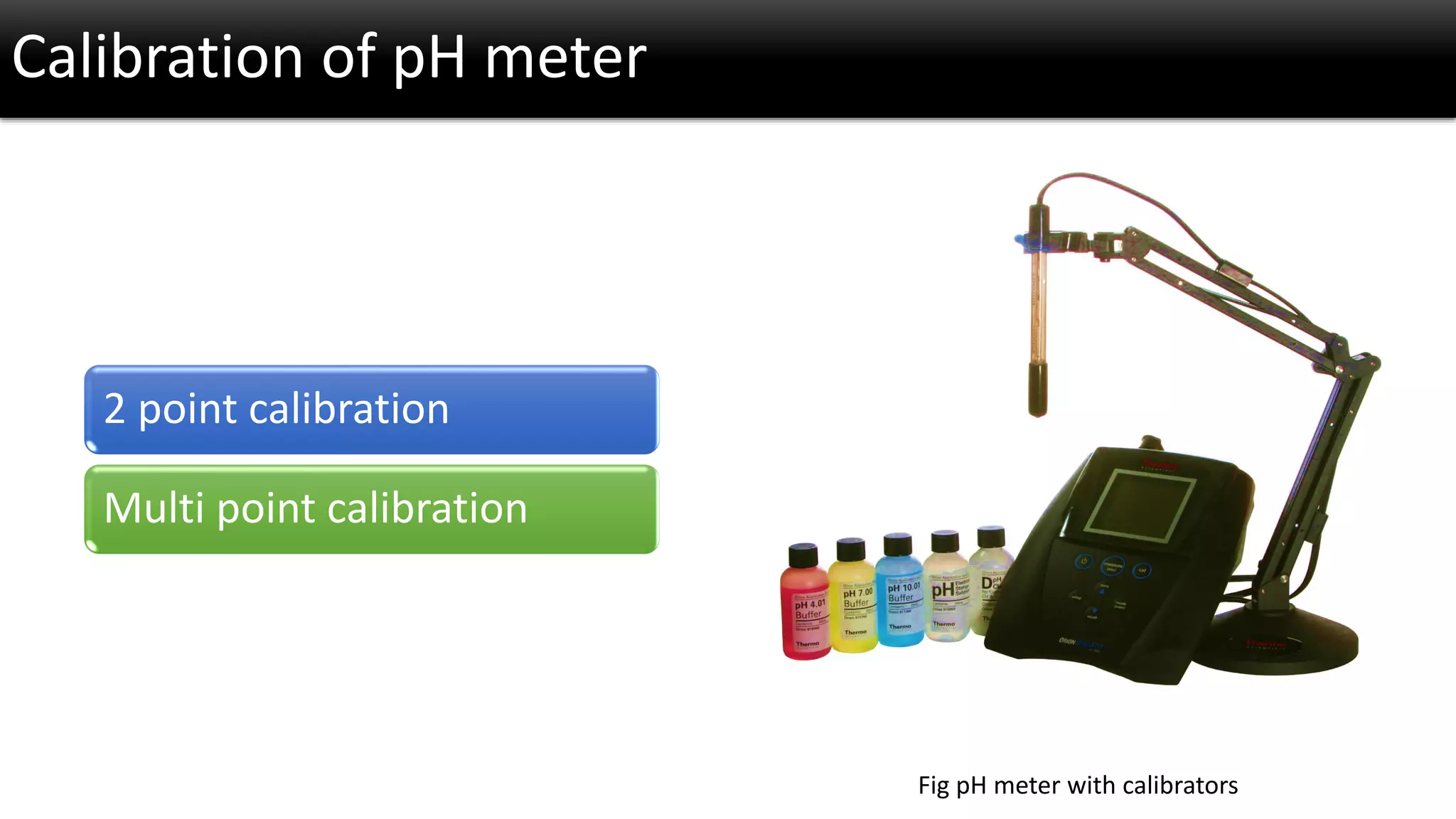
The document discusses the concept of pH and its measurement, detailing the historical origin of pH and methods for determining it, such as using pH meters and electrodes. It covers calibration processes, errors in pH determination, and factors that affect electrode life and measurement accuracy. The document also addresses the definition of pH in terms of hydrogen ion concentration and activity, along with references for further reading.
![Origin of pH
SørenP. L. Sörenson
(1868-1939)
H+
H+
H+
H+
Number of Hydrogen ions (H+) determine acidity or alkalinity
•Number of Hydrogen ions (H+) in water = 0.0000001 mol/L
•Logarithm of H+Concentration
•Log (0.0000001) = log (10-7) = -7
•Negative Logarithm of H+Concentration
•-[Log (0.0000001)] = -log (10-7) = -(-7) = 7
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
pH Scale
Alkaline
Acidic
Power of Hydrogen
p
H](https://image.slidesharecdn.com/basicsofphitsmeasurementslideshare-140909024224-phpapp01/75/pH-its-measurement-2-2048.jpg)