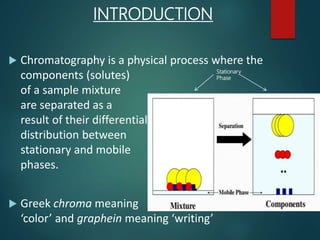
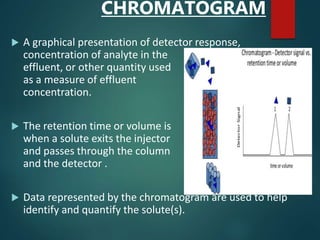
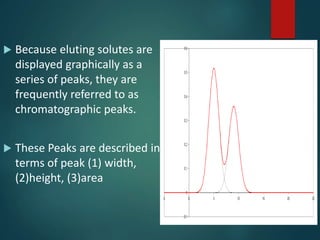
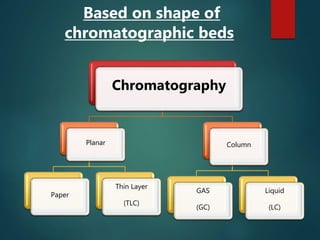
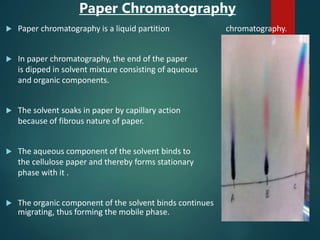
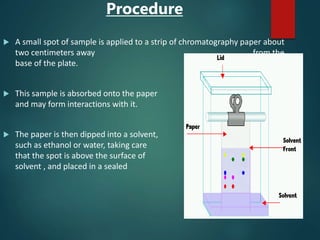
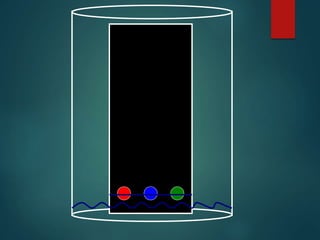
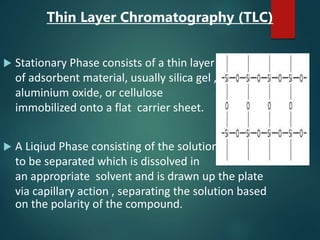
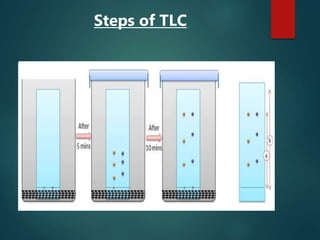
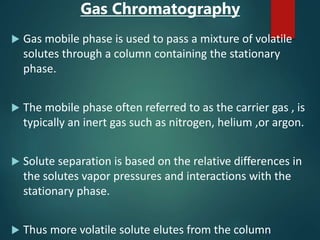
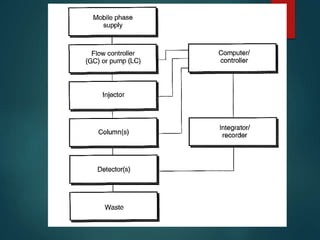
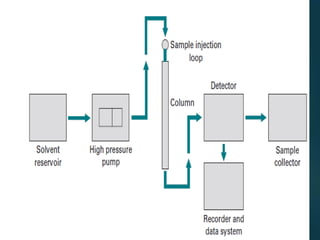
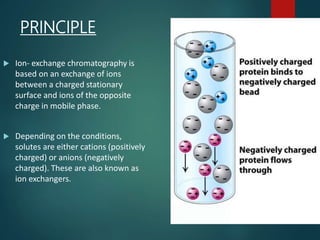
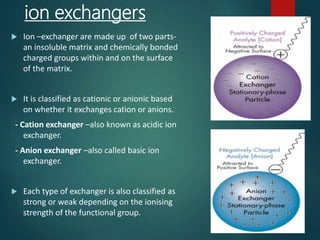
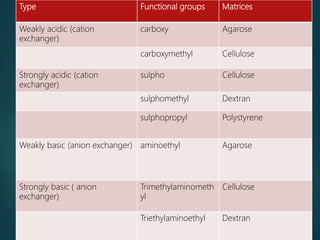
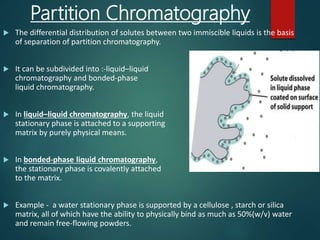
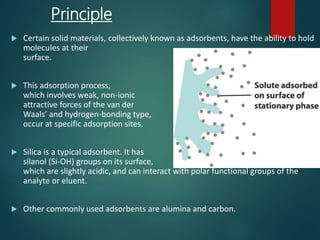
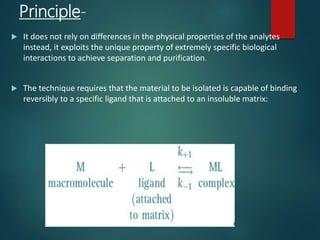
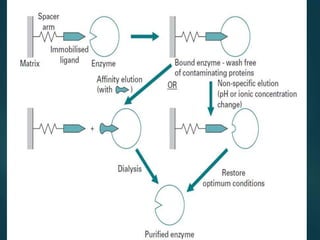
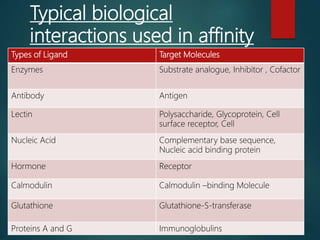
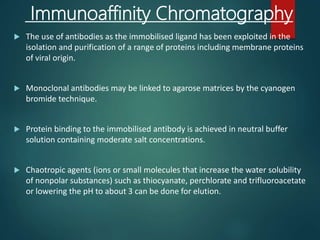
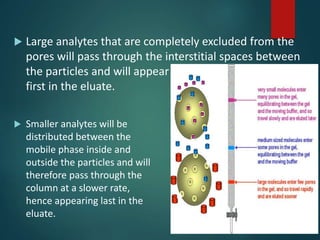
Chromatography is a technique used to separate the components of a mixture through differential partitioning between a stationary and mobile phase. There are various types of chromatography classified by the physical state of the phases used and the separation mechanism employed. The document discusses the basic principles and history of chromatography. It describes different techniques like paper chromatography, thin layer chromatography, gas chromatography, liquid chromatography and ion exchange chromatography. Applications and significance of these techniques in fields like pharmaceuticals, forensics and food analysis are also highlighted.