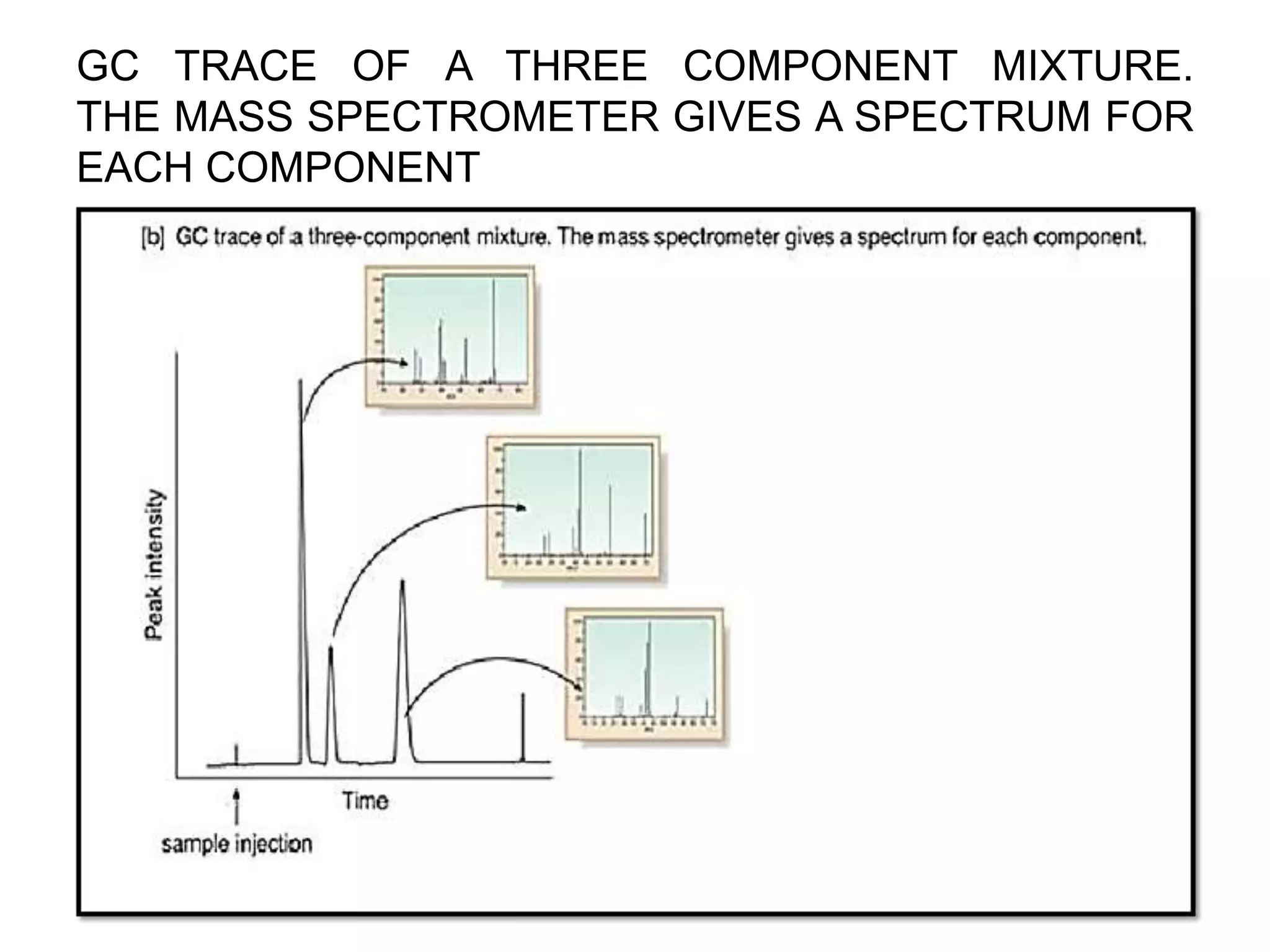
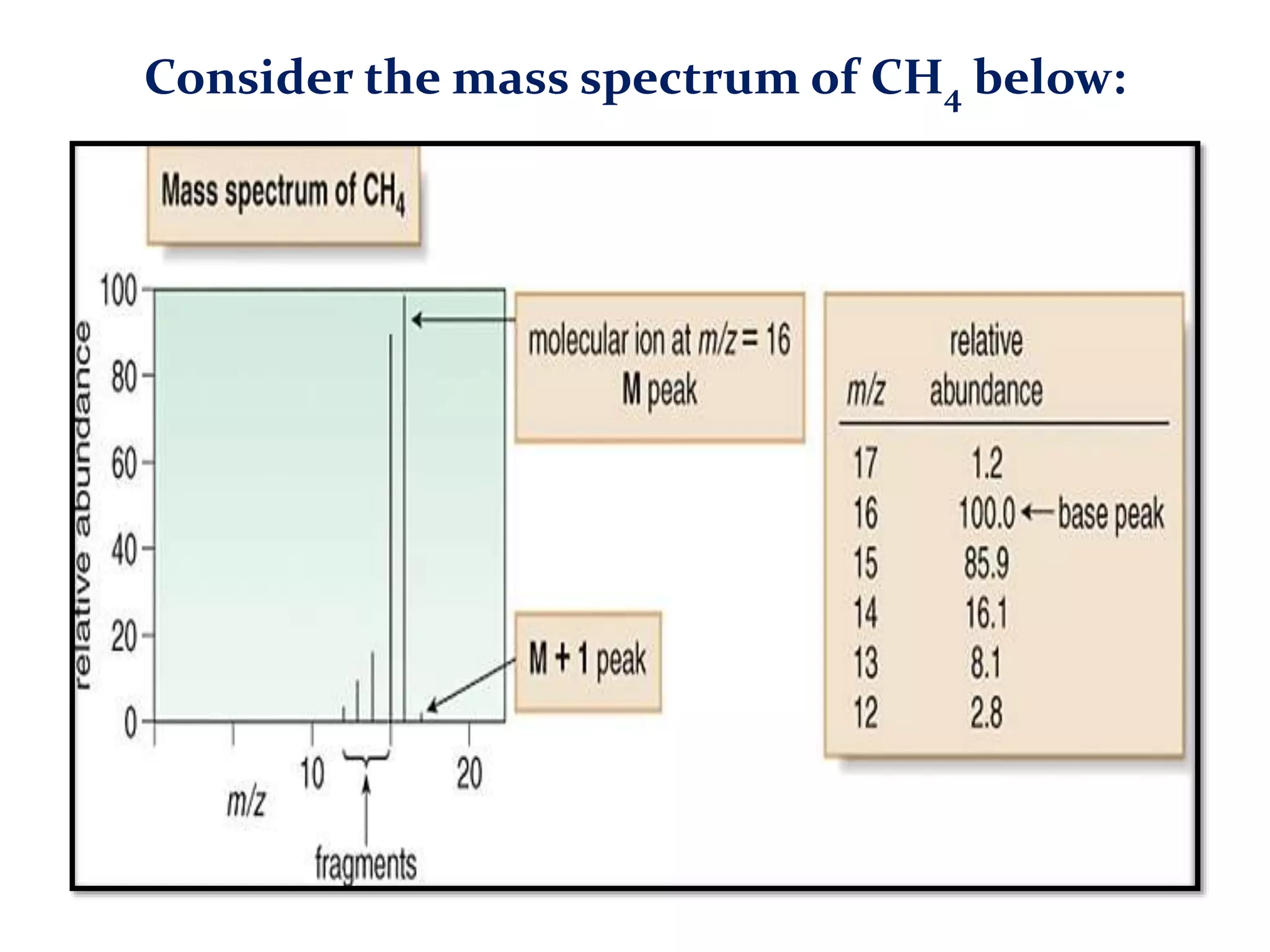
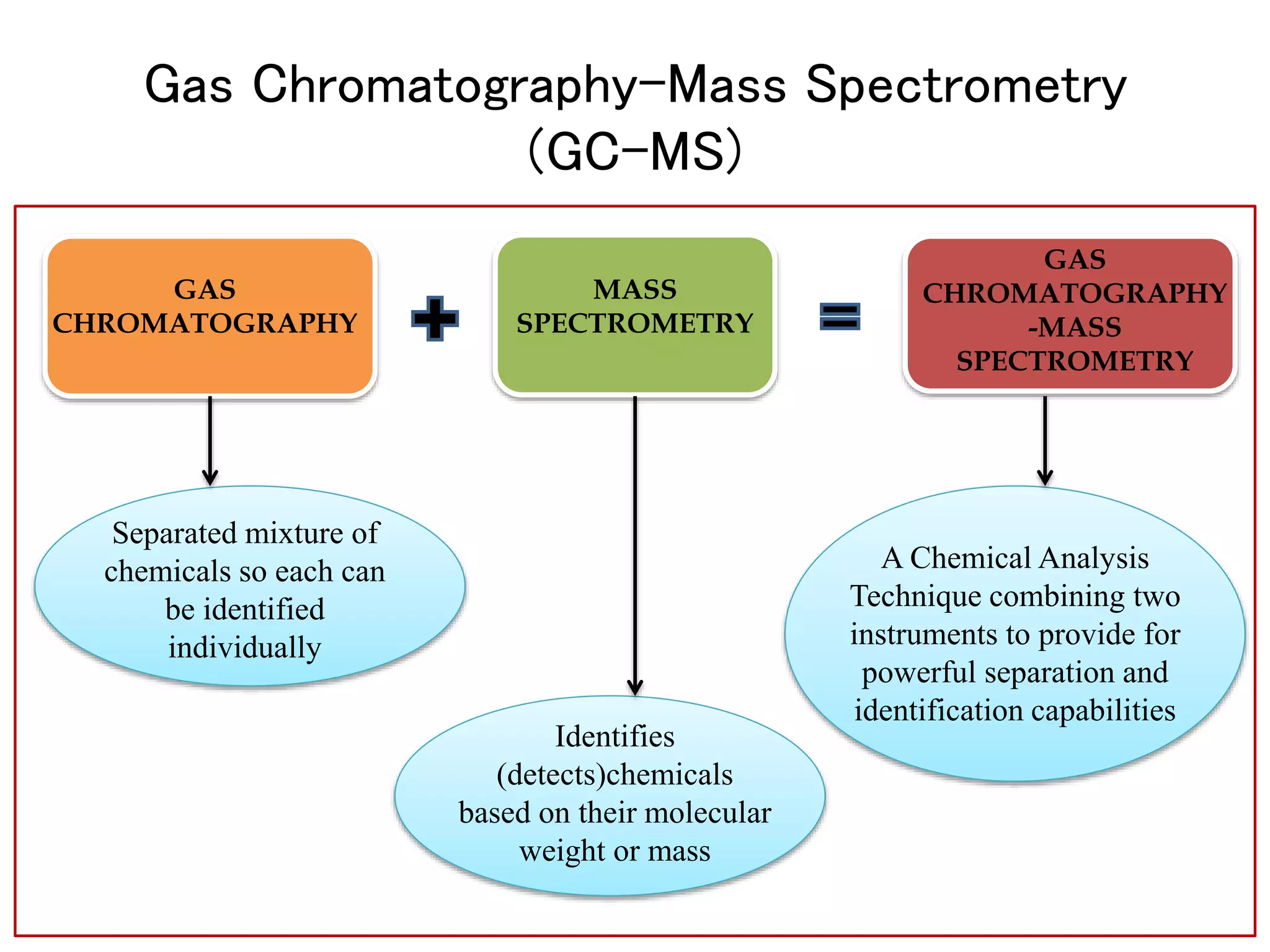
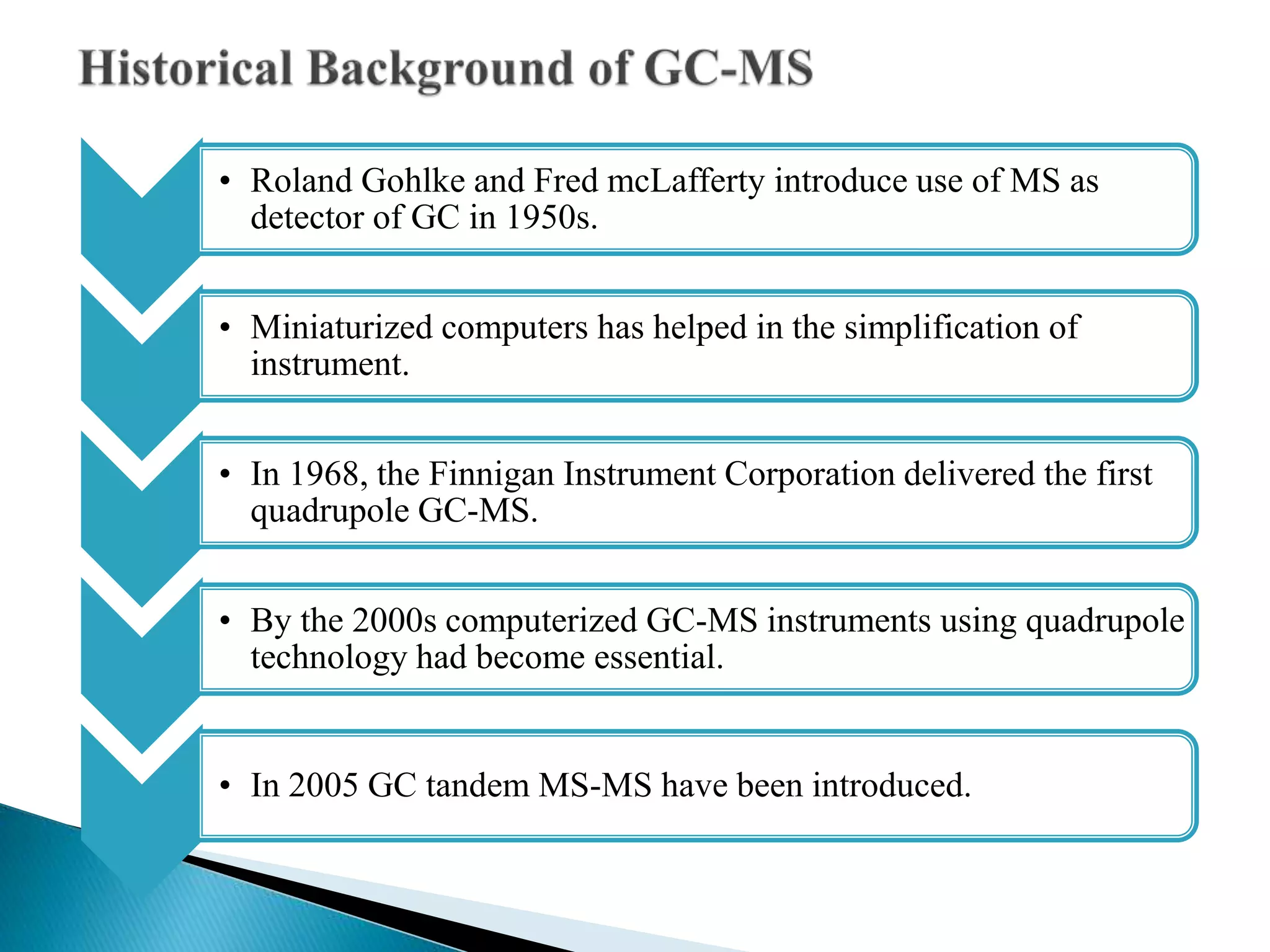
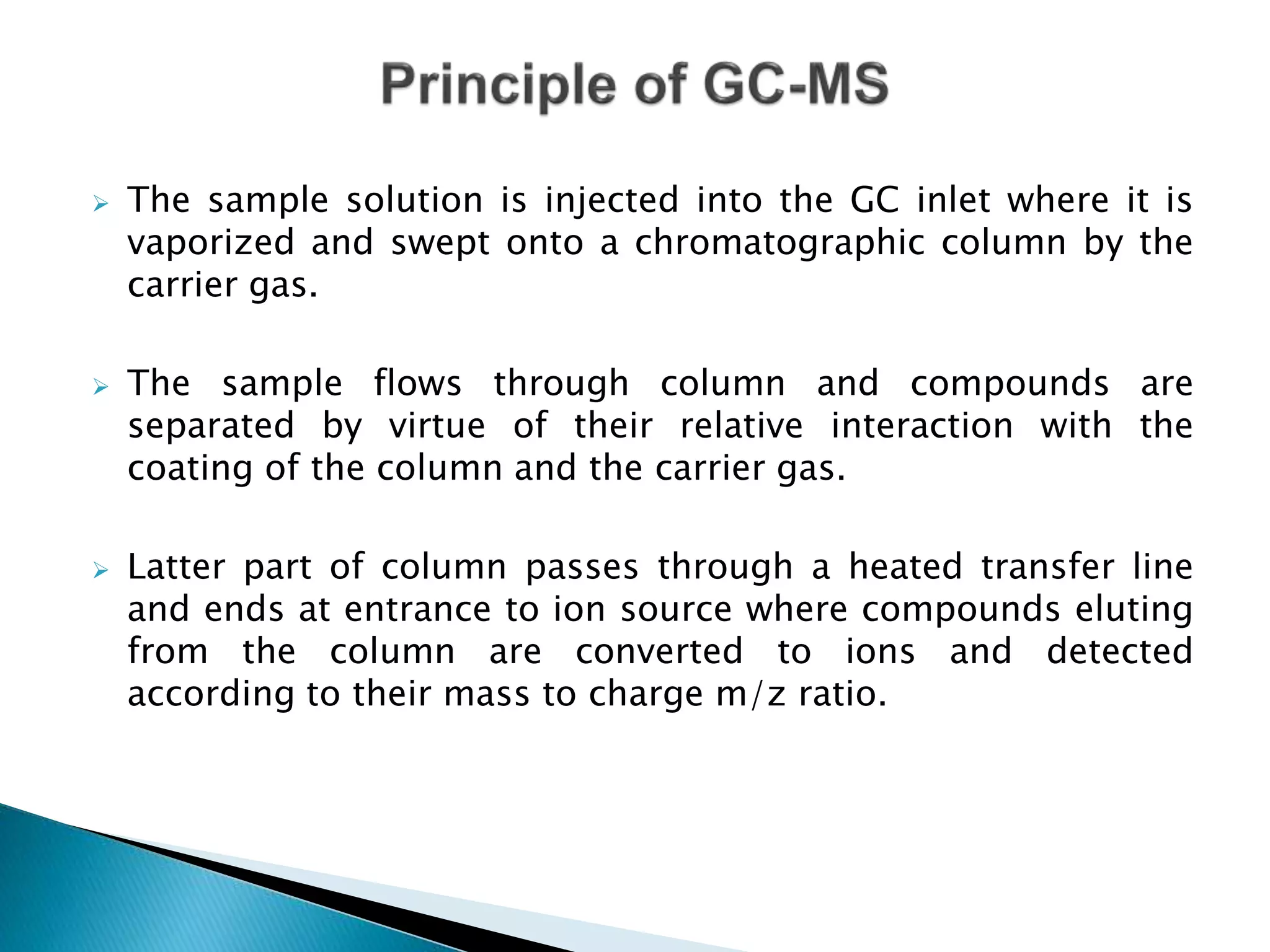
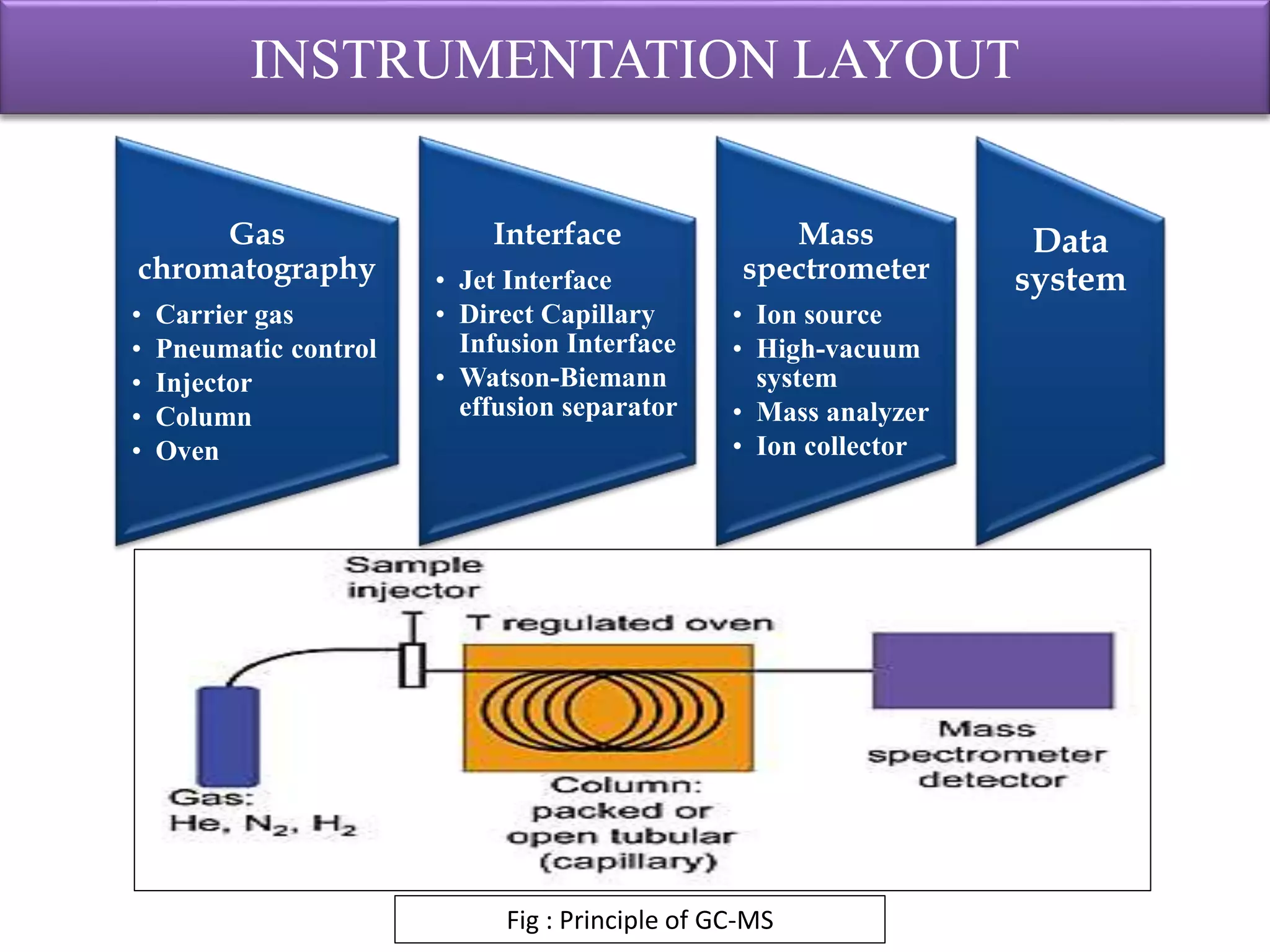
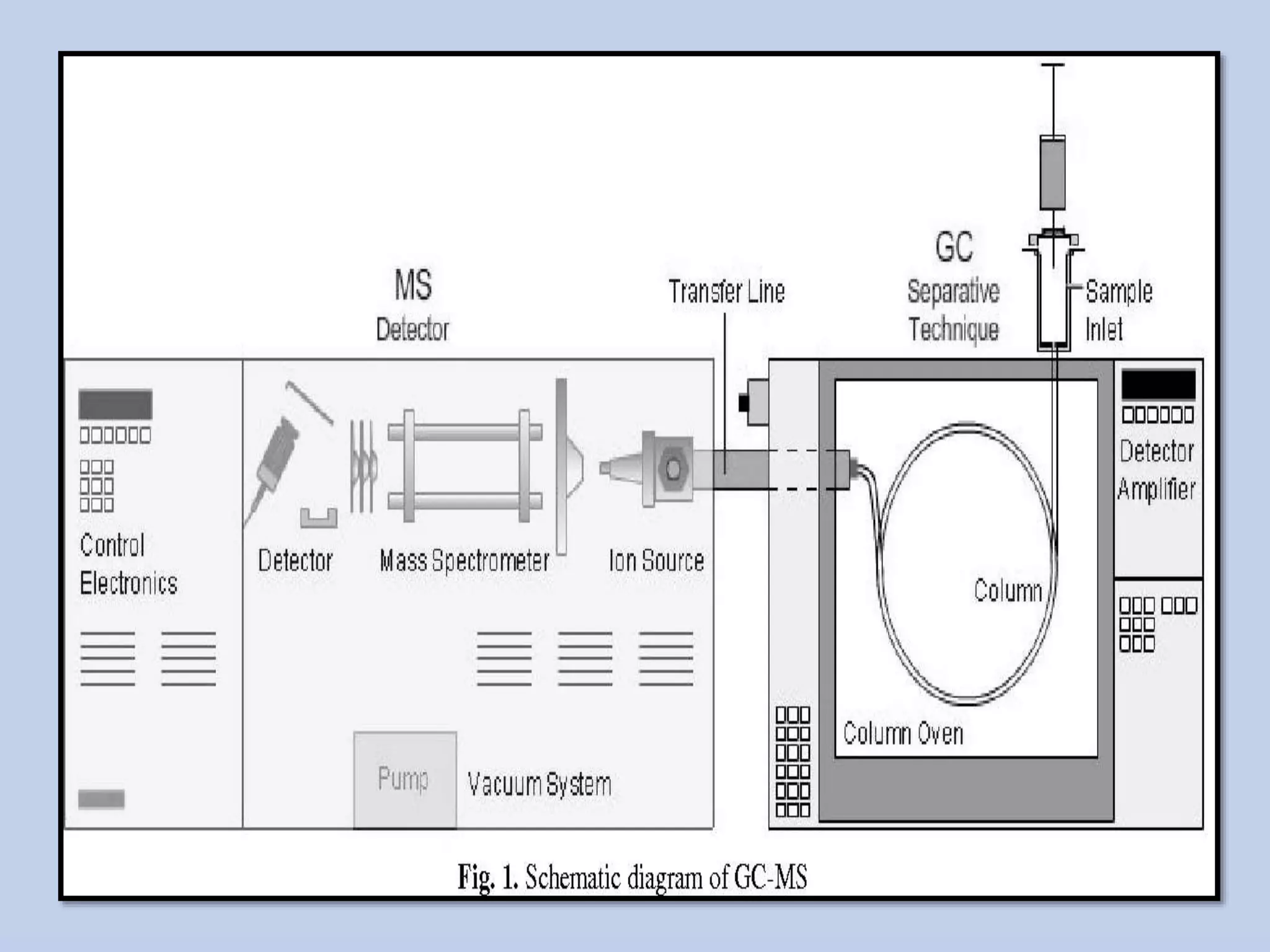
The document provides an in-depth overview of Gas Chromatography-Mass Spectrometry (GC-MS), detailing its principles, instrumentation, and applications. It discusses the historical development, key components involved in the analysis, and the strengths and limitations of the GC-MS technique. Applications of GC-MS span various fields including forensics, environmental analysis, and clinical toxicology, emphasizing its importance in both qualitative and quantitative measurements.






![Ionization Method Typical Analytes Sample
Introduction
Mass
Range
Method
Highlights
Chemical Ionization (CI) Relatively small,
volatile
GC or
liquid/solid
probe
Upto
1000
Daltons
Soft method,
molecular ion peak
[M+H] +
Electron Impact
Ionization (EI)
Relatively small,
volatile
GC or
liquid/solid
probe
Upto
1000
Daltons
Hard method,
versatile, provides
structure info
Electrospray Ionization
(ESI)
Peptides, proteins,
nonvolatile
Liquid
chromatography
Upto
200000
Daltons
Soft method, ions
often multiply
charged
Fast Atom Bombardment
(FAB)
Carbohydrates,
organometallics,
peptides,
nonvolatile
Sample mixed in
viscous matrix
Upto
6000
Daltons
Harder than ESI or
MALDI
Matrix Assisted Laser
Desorption Ionization
(MALDI)
Peptides, proteins,
nucleotides
Sample mixed in
solid matrix
Upto
500000
Daltons
Soft method, very
high mass](https://image.slidesharecdn.com/gaschromatography-massspectrometrygc-ms-191115202105/75/Gas-chromatography-mass-spectrometry-GC-MS-16-2048.jpg)