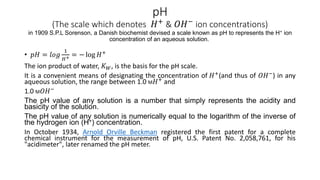
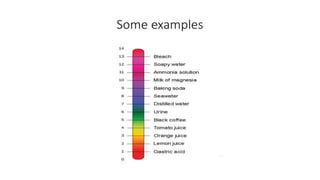
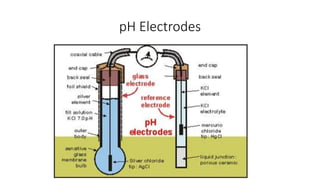
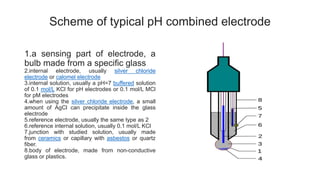
The document covers the principles and uses of various biological tools and techniques, particularly focusing on pH meters used for measuring acidity and alkalinity in solutions. It explains the pH scale, the design and functioning of electrodes, including glass and reference electrodes, and details the calibration and maintenance of pH meters. Additionally, it highlights the various cleaning methods for electrodes and references textbooks for further reading.