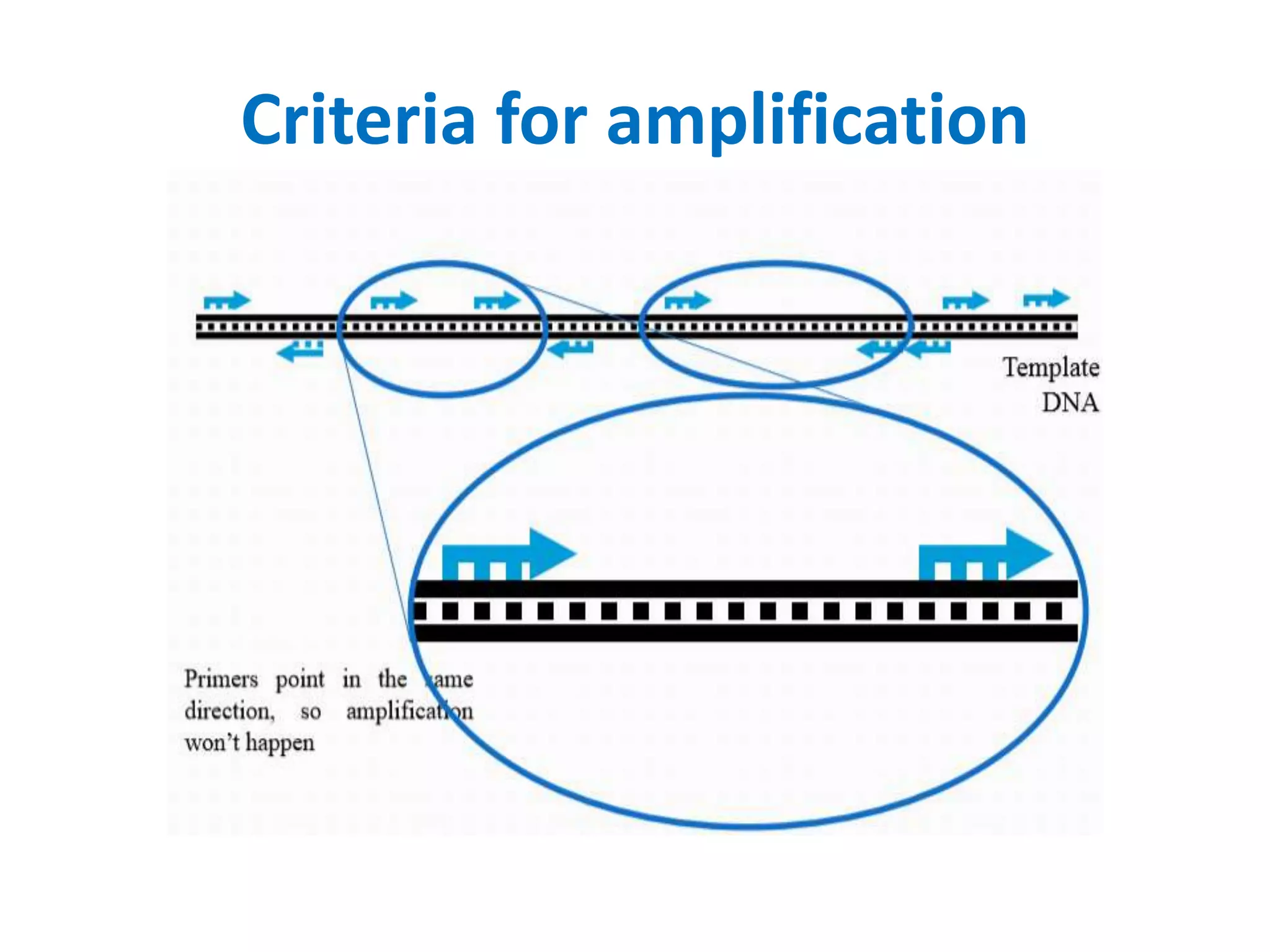
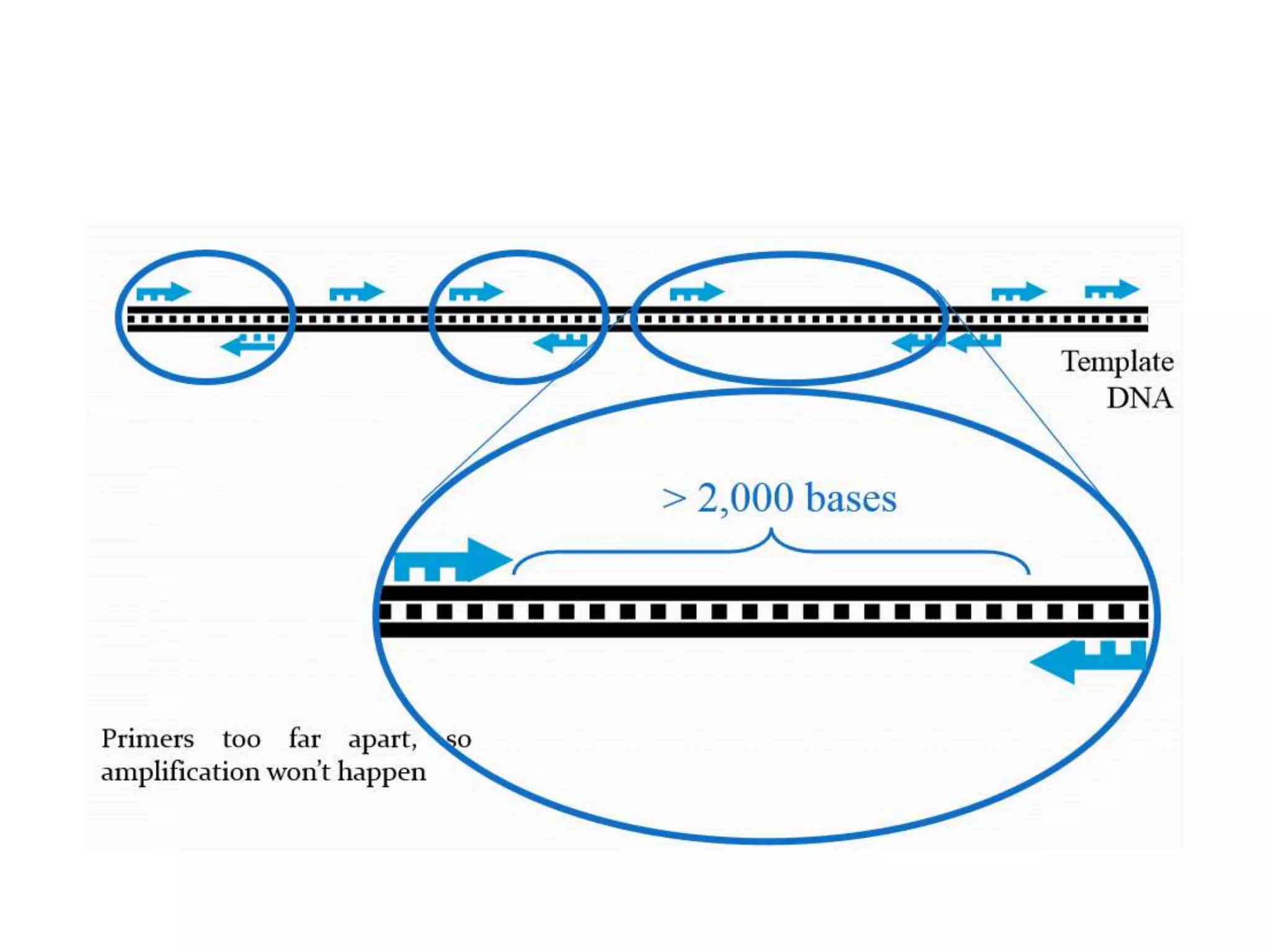
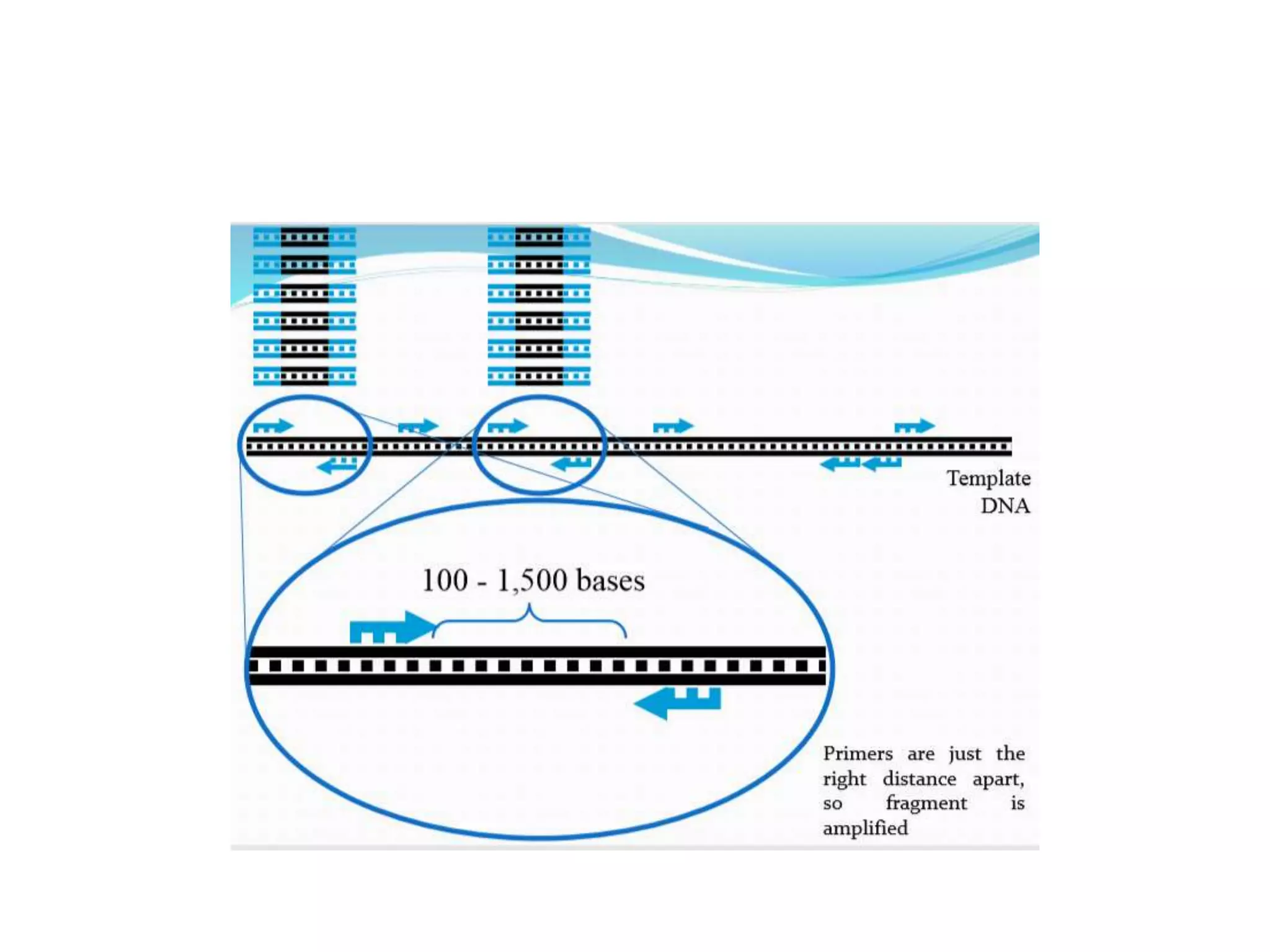
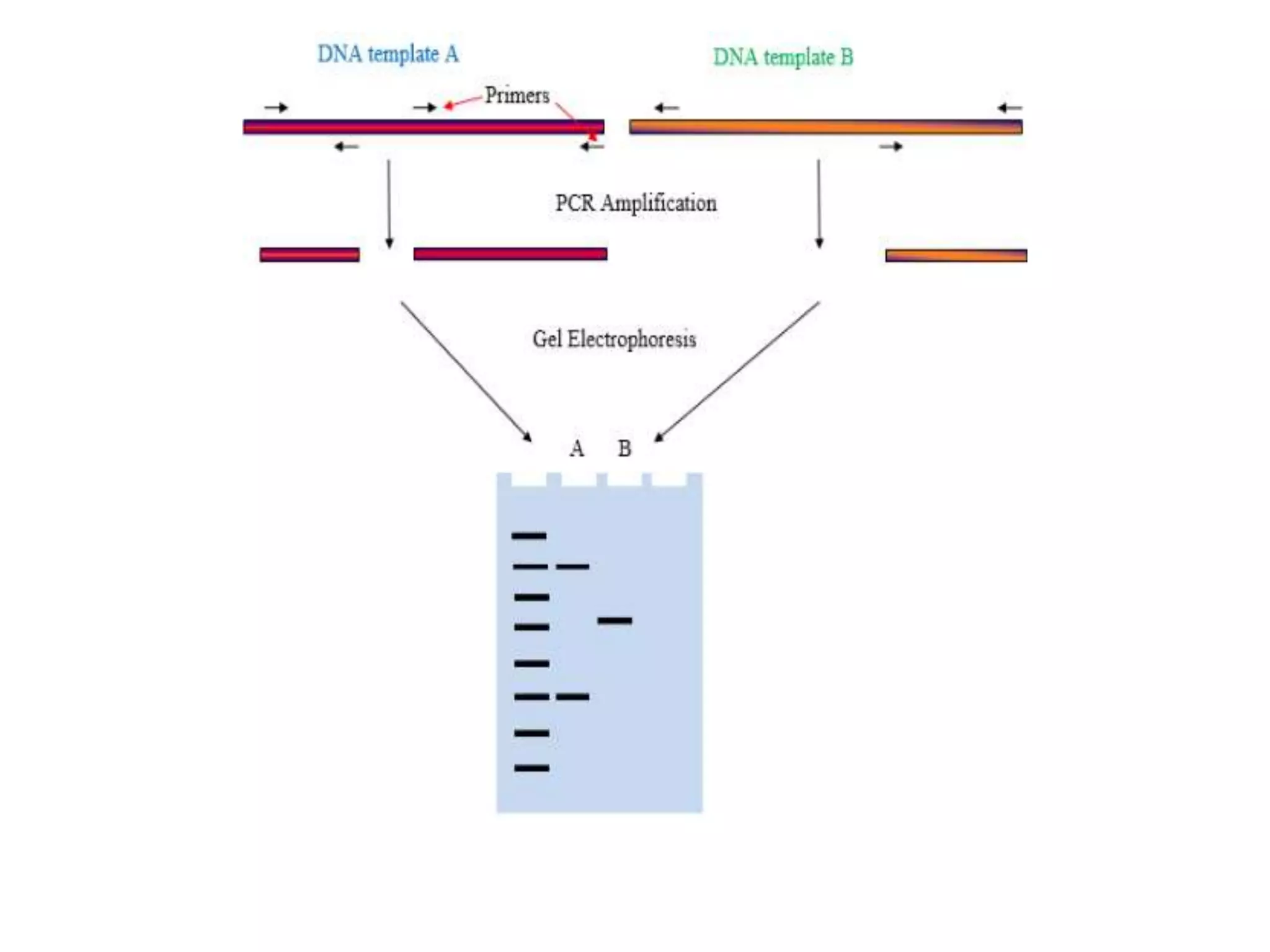
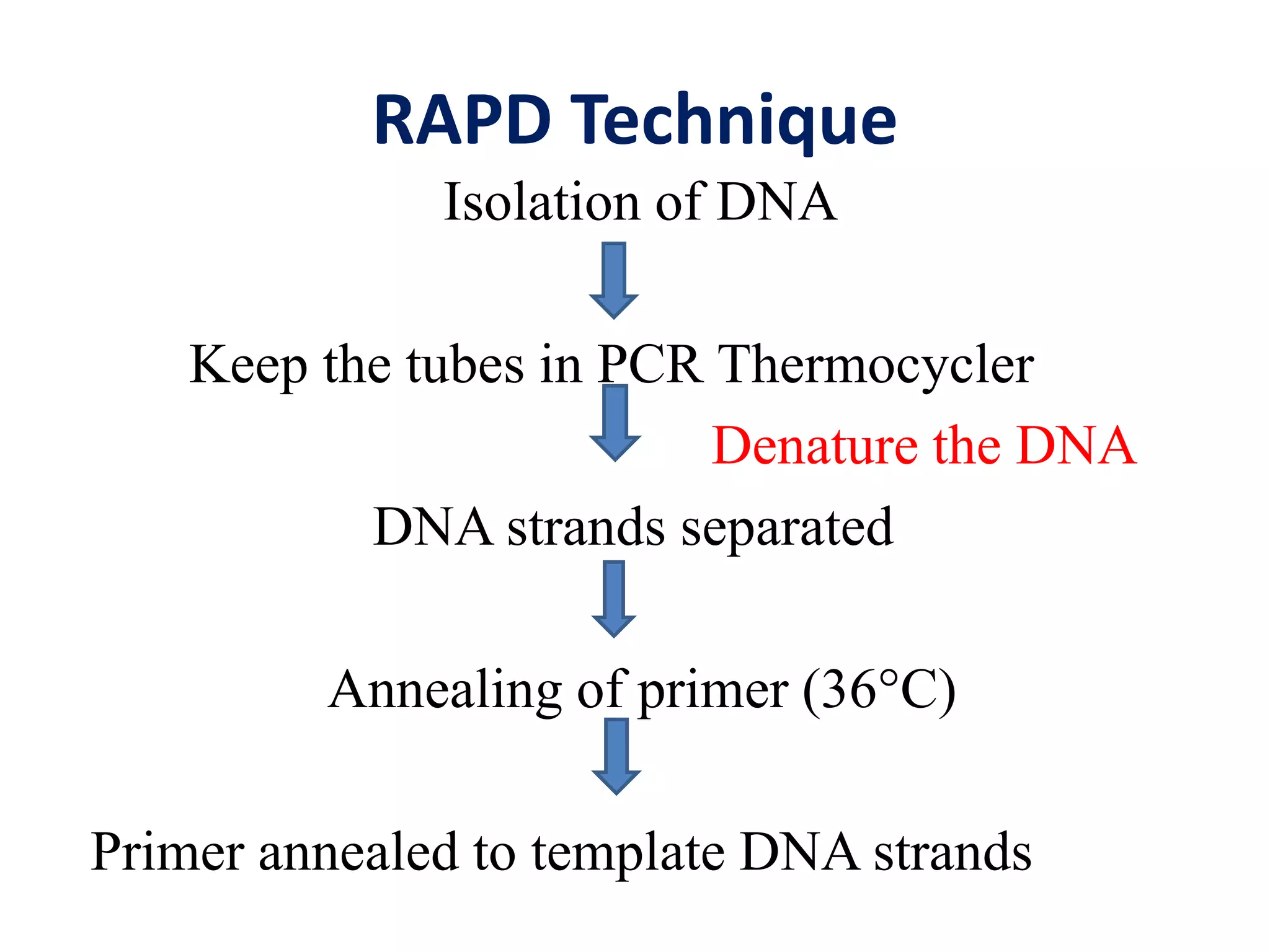
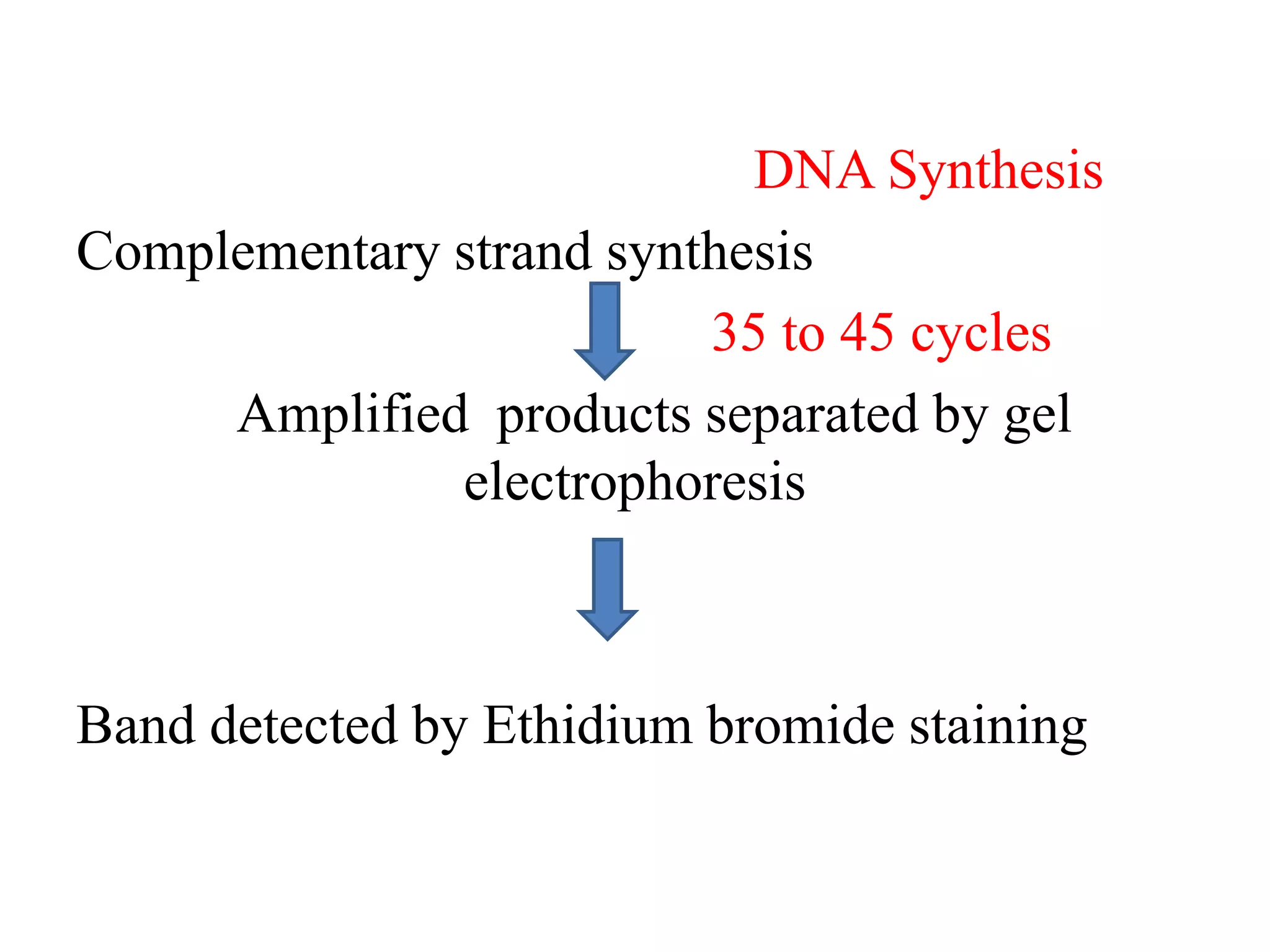
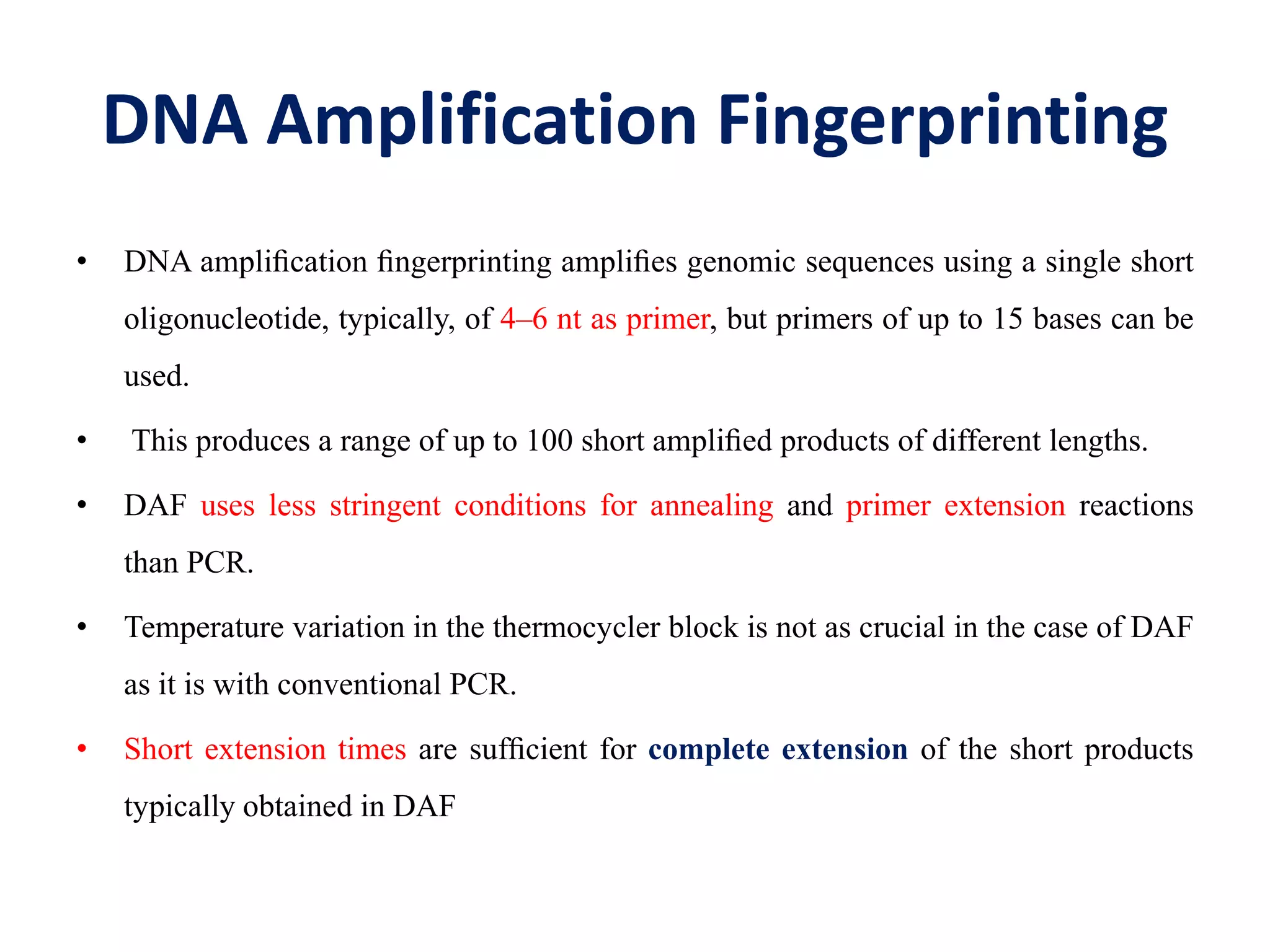
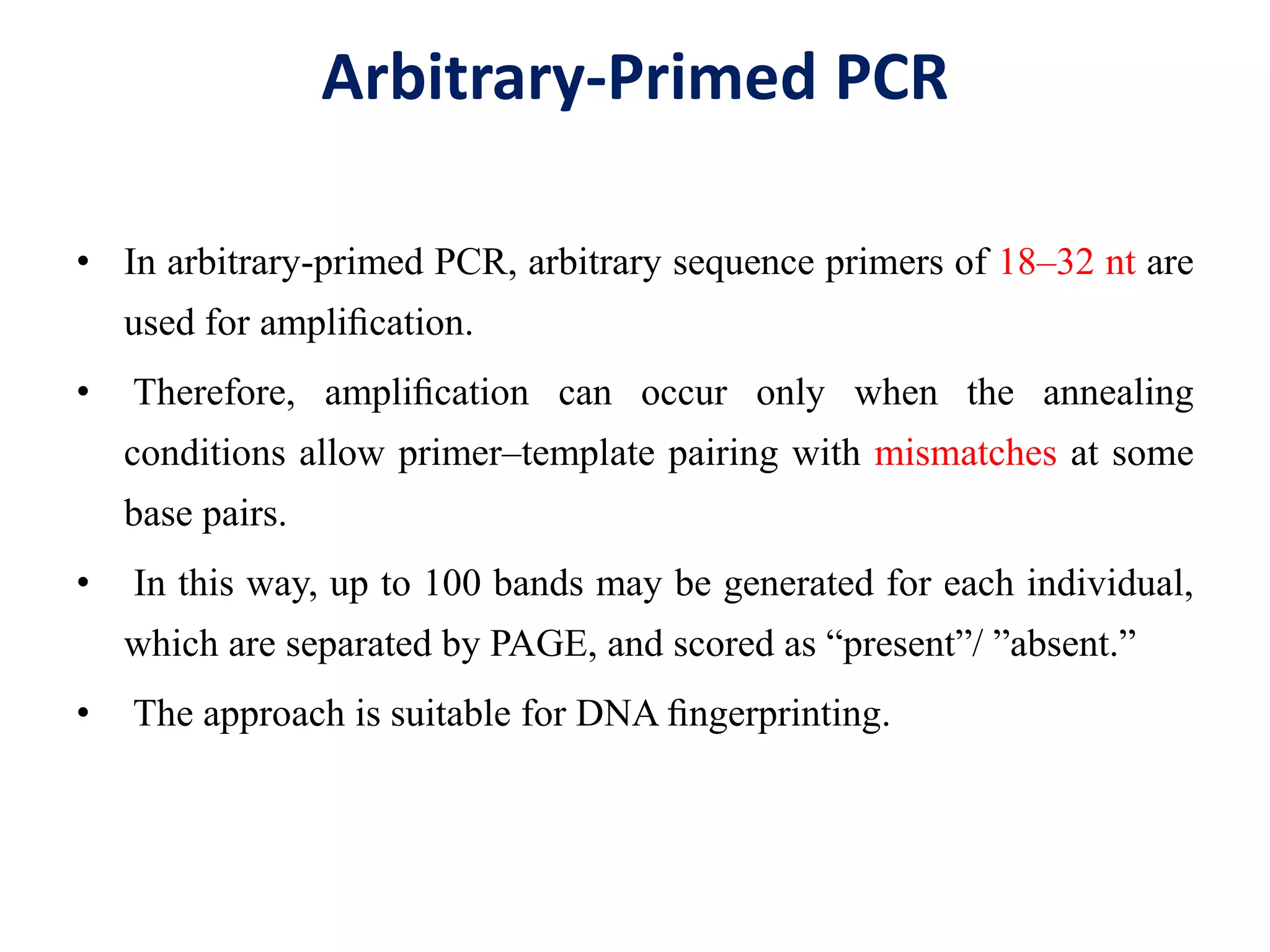
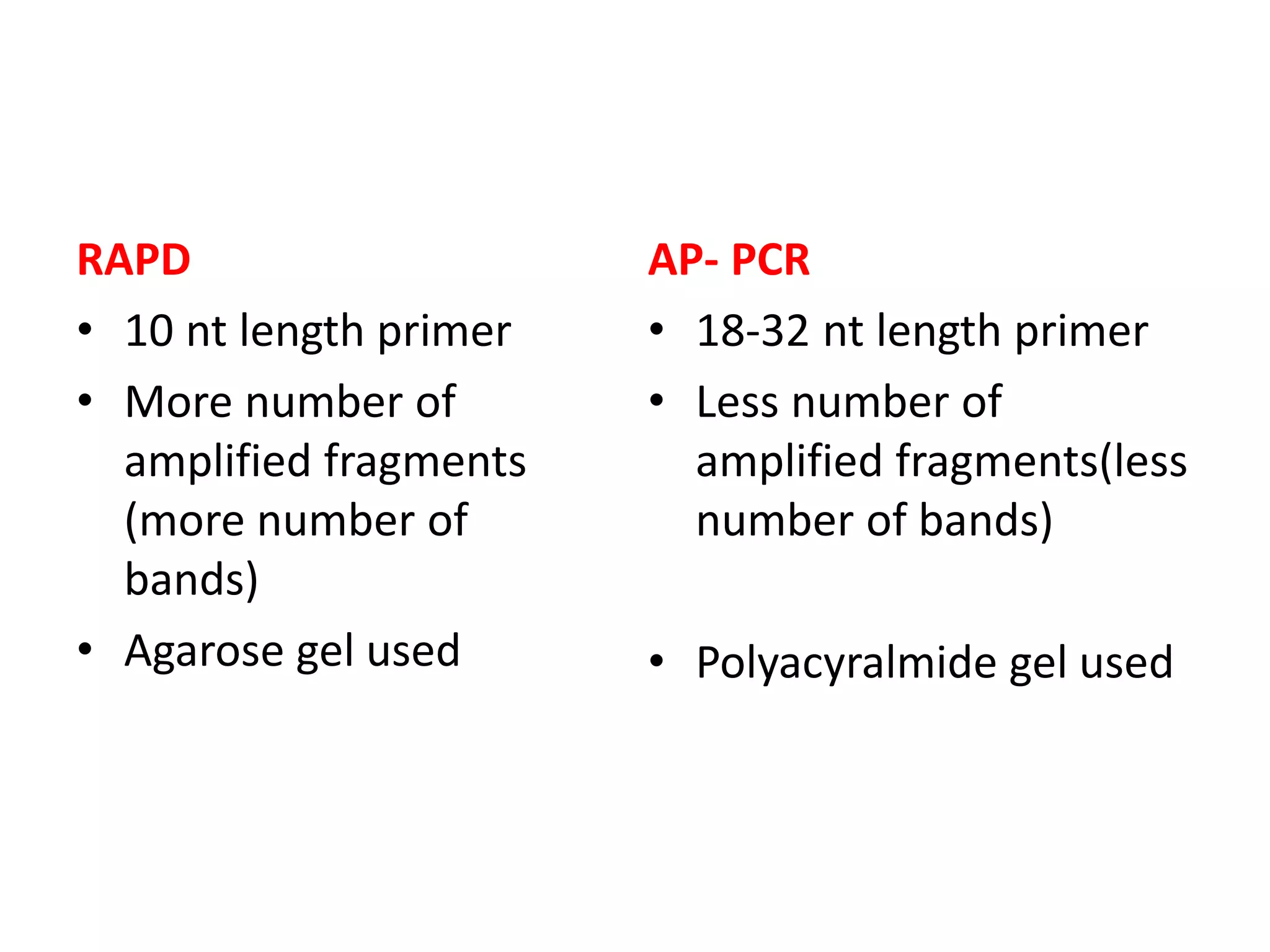
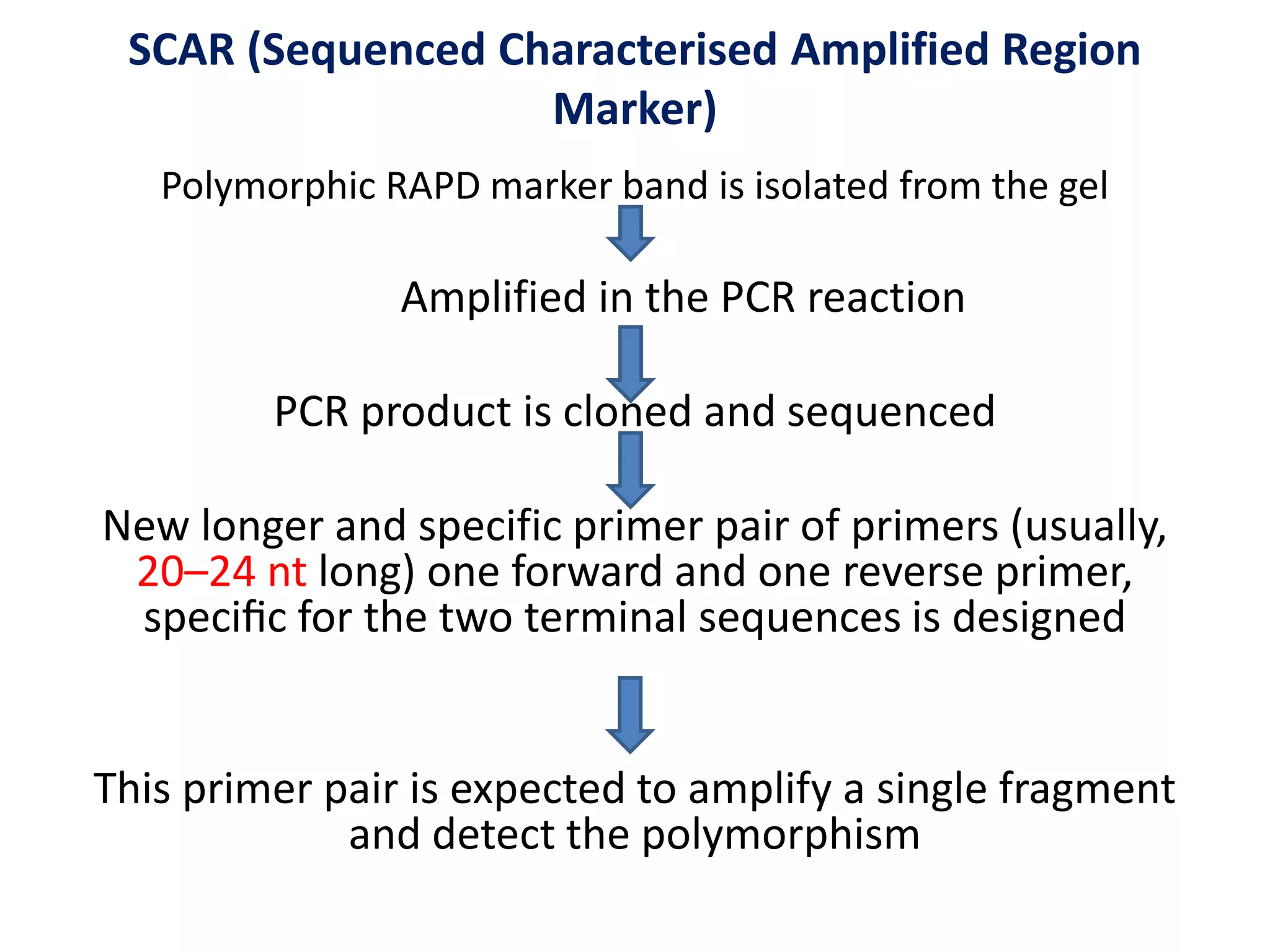
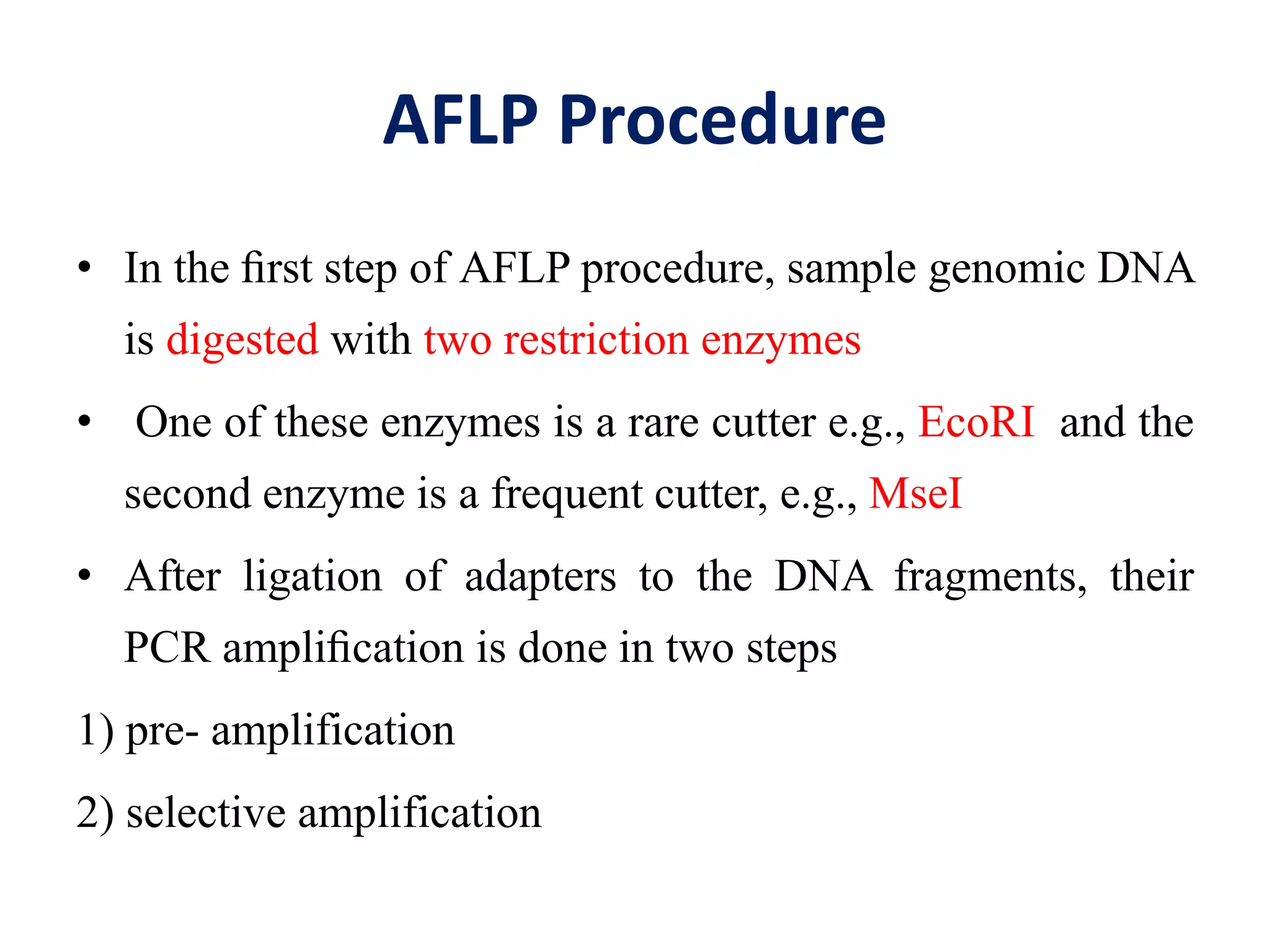
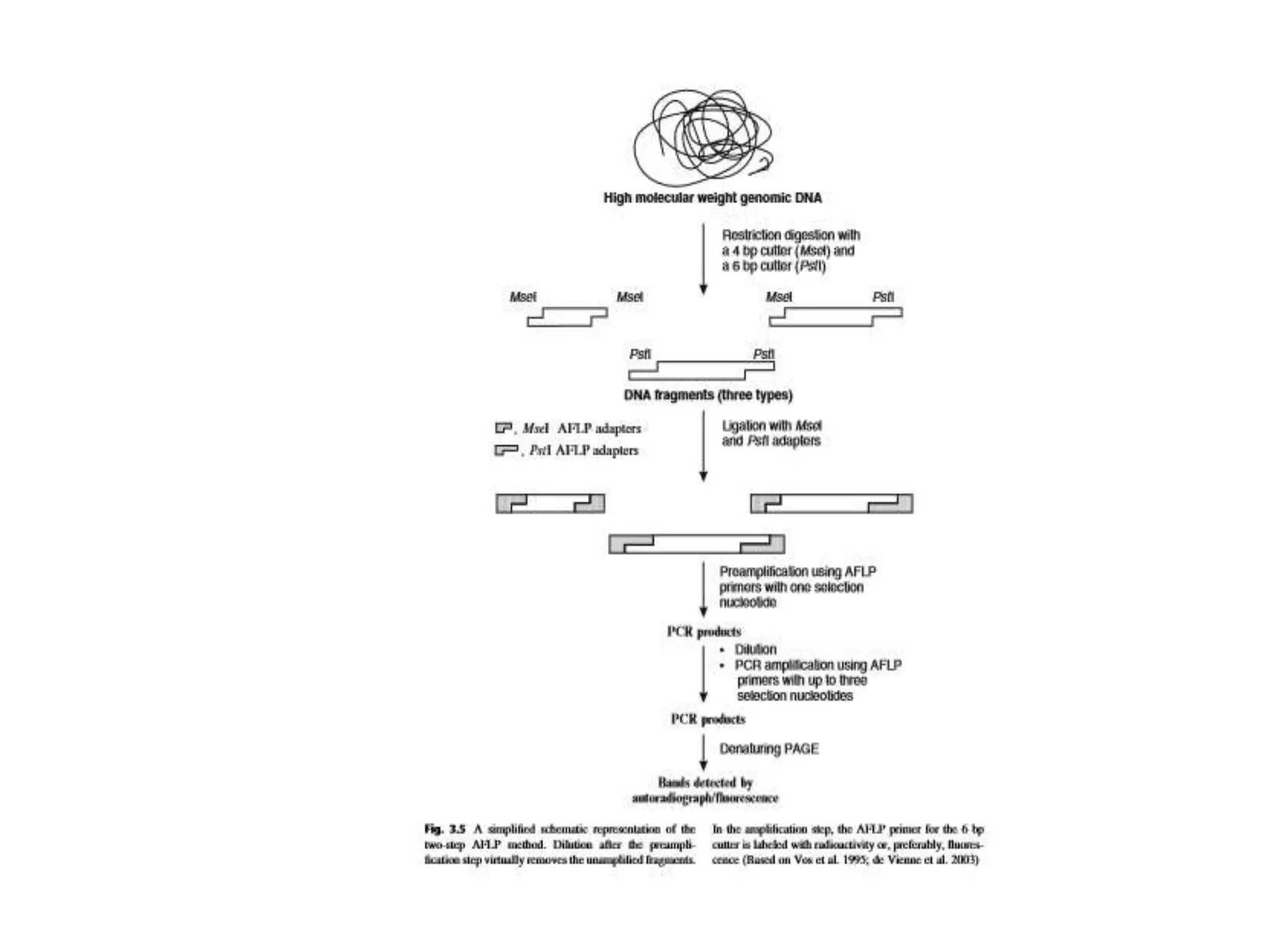
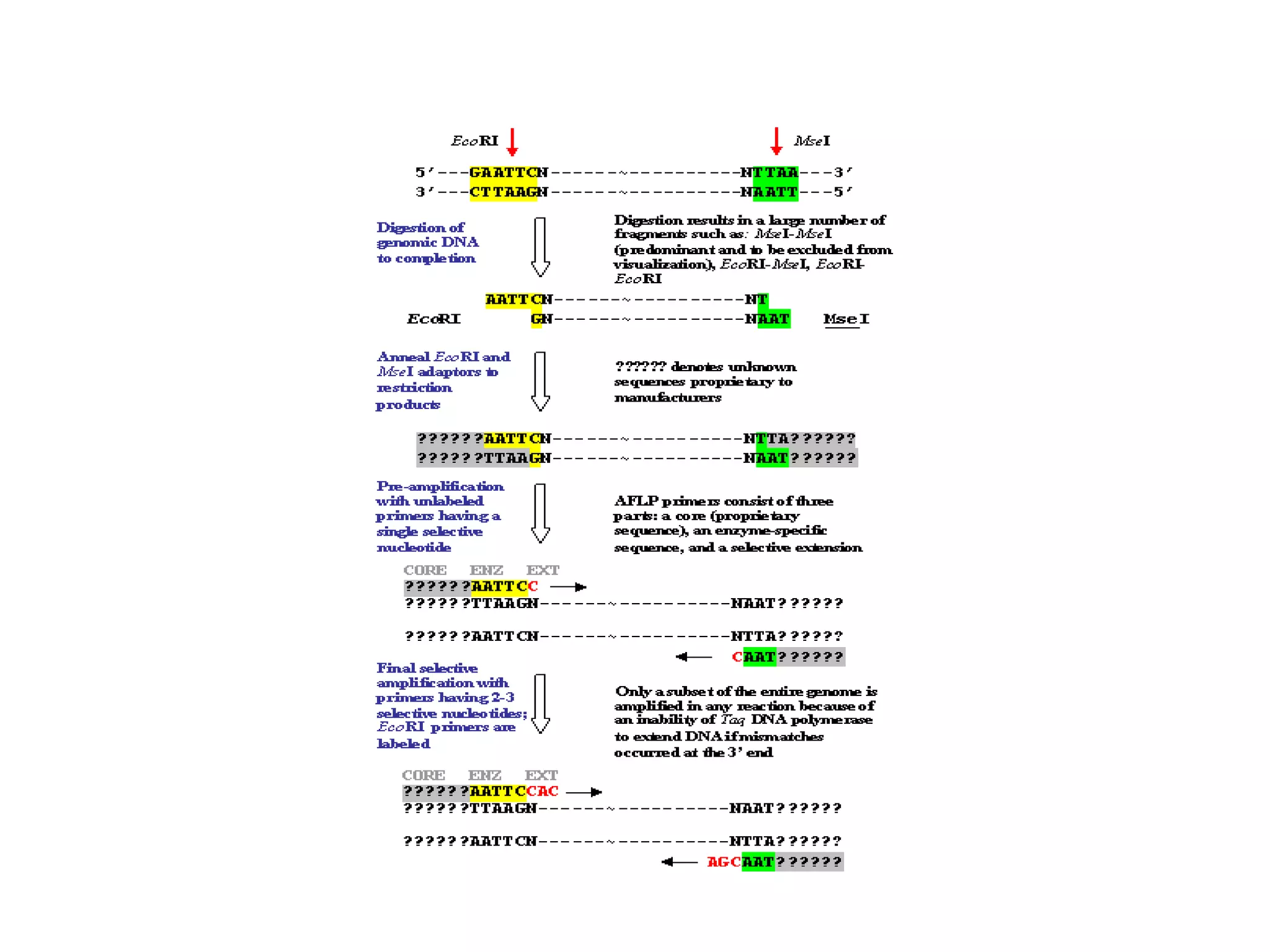
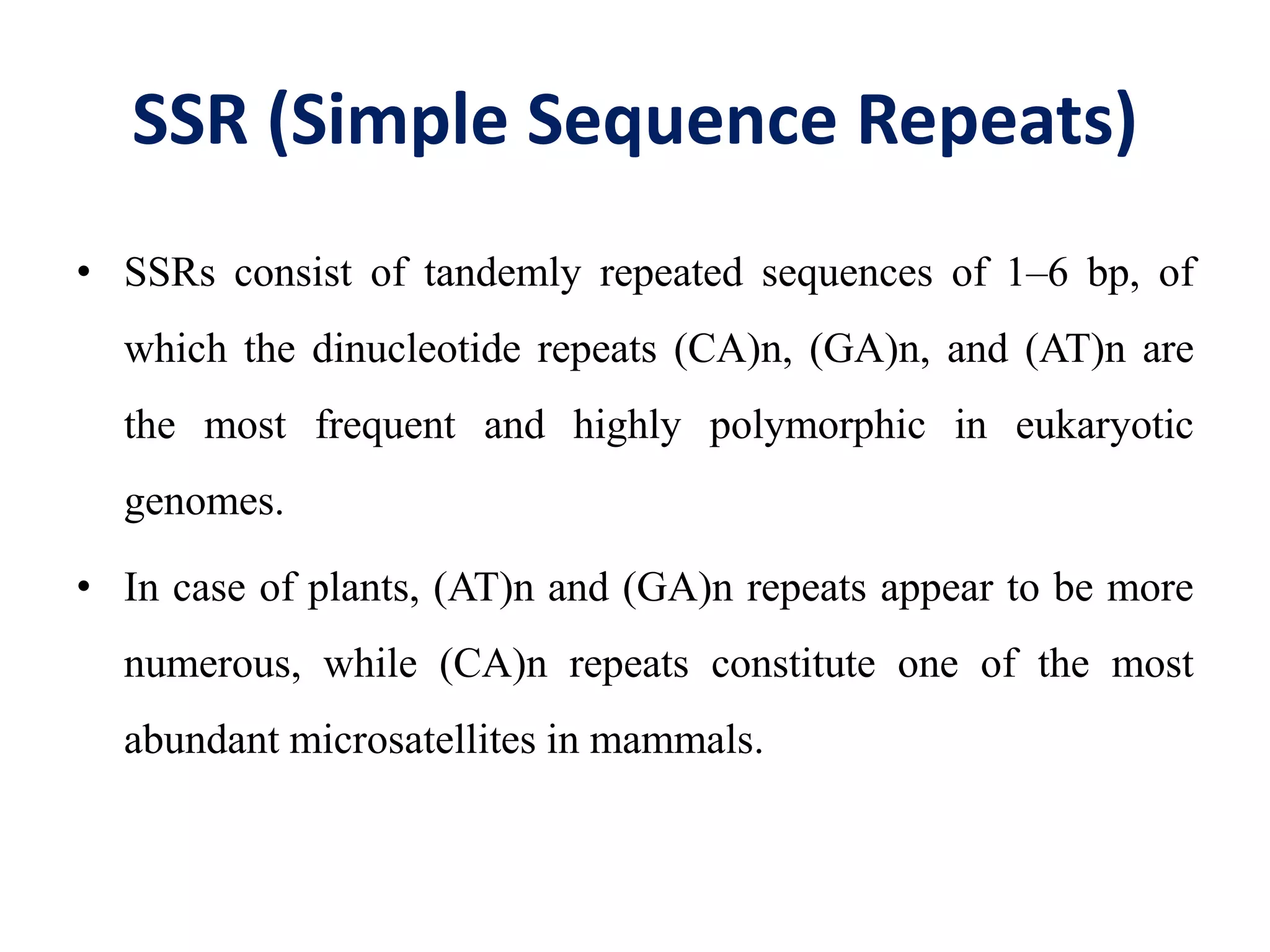
This document discusses various types of PCR-based molecular markers used in genetic analysis. It begins by describing PCR and how it is used to amplify DNA fragments. It then explains different steps of PCR like denaturation, annealing and extension. The document further discusses different types of PCR like reverse transcription PCR, real-time PCR, multiplex PCR etc. It also summarizes various molecular marker techniques derived from PCR including RAPD, AFLP, SSR, SCAR etc and explains the procedure involved in each.