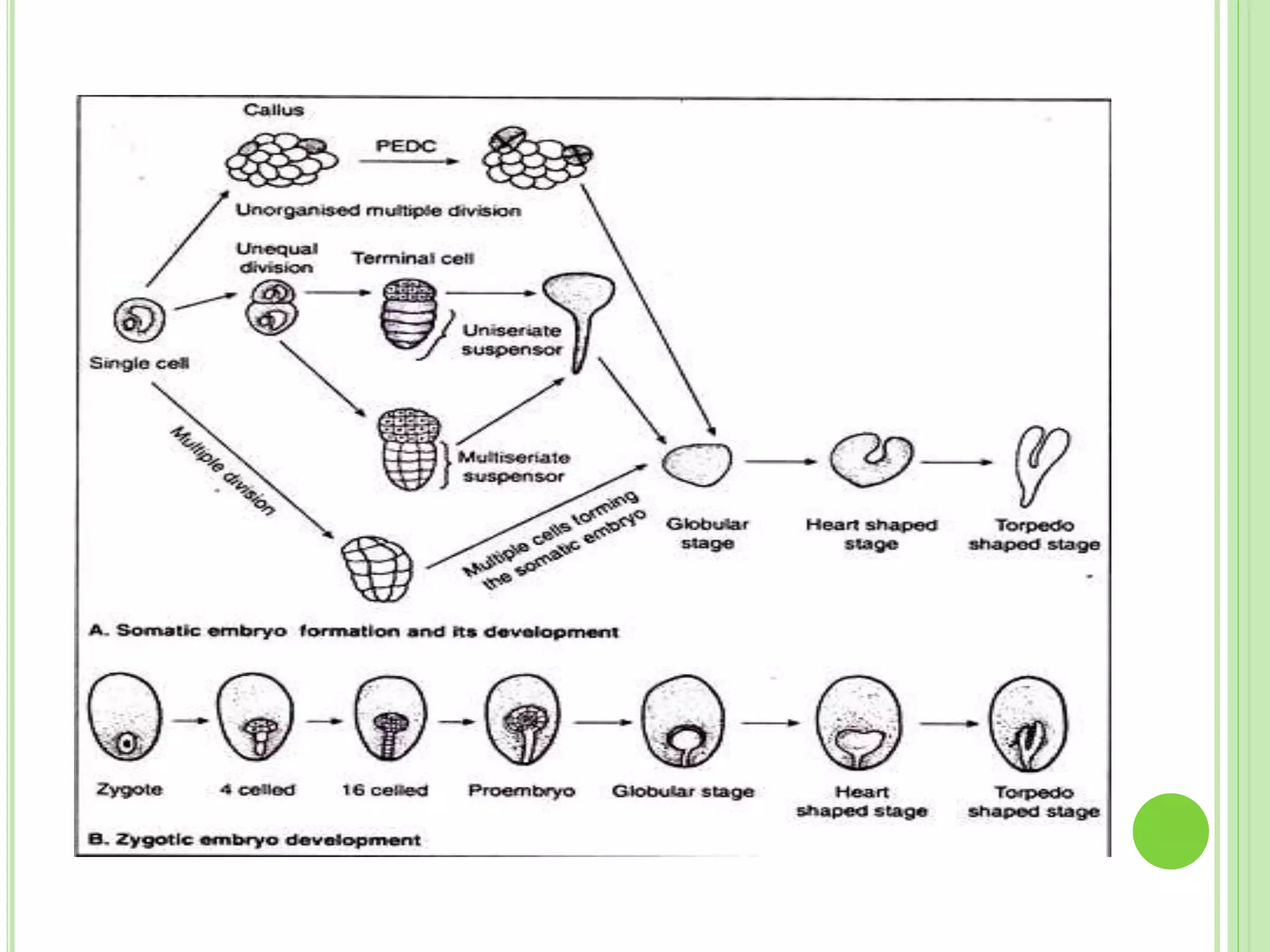
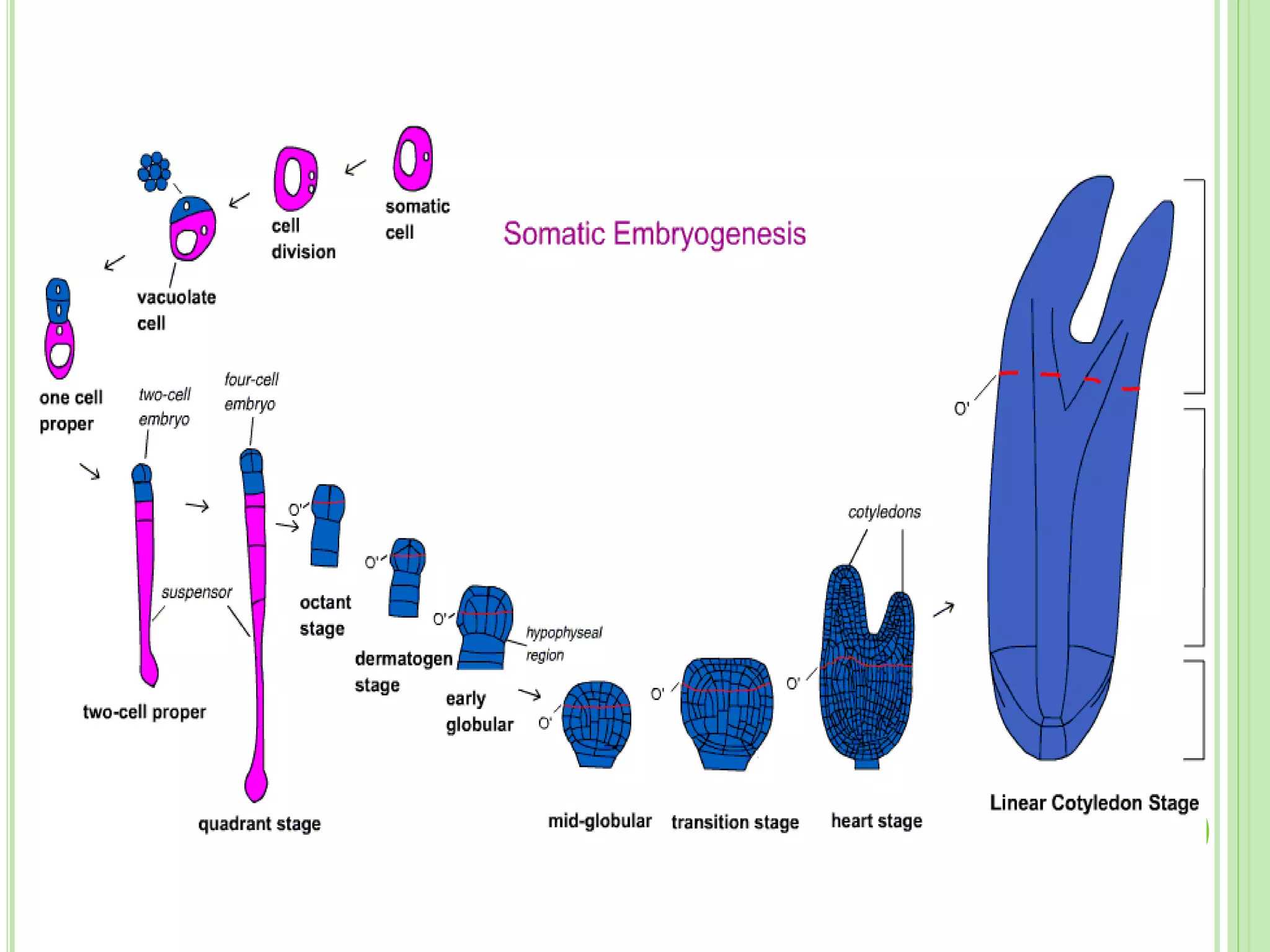
The document discusses somatic embryogenesis, a process in plant tissue culture where embryos develop from vegetative cells rather than fertilized eggs. It covers the history, development stages, factors affecting embryogenesis, and applications like synthetic seed production and genetic engineering. The text highlights the significance of growth regulators, explants, and genotype in influencing the efficiency of somatic embryogenesis.