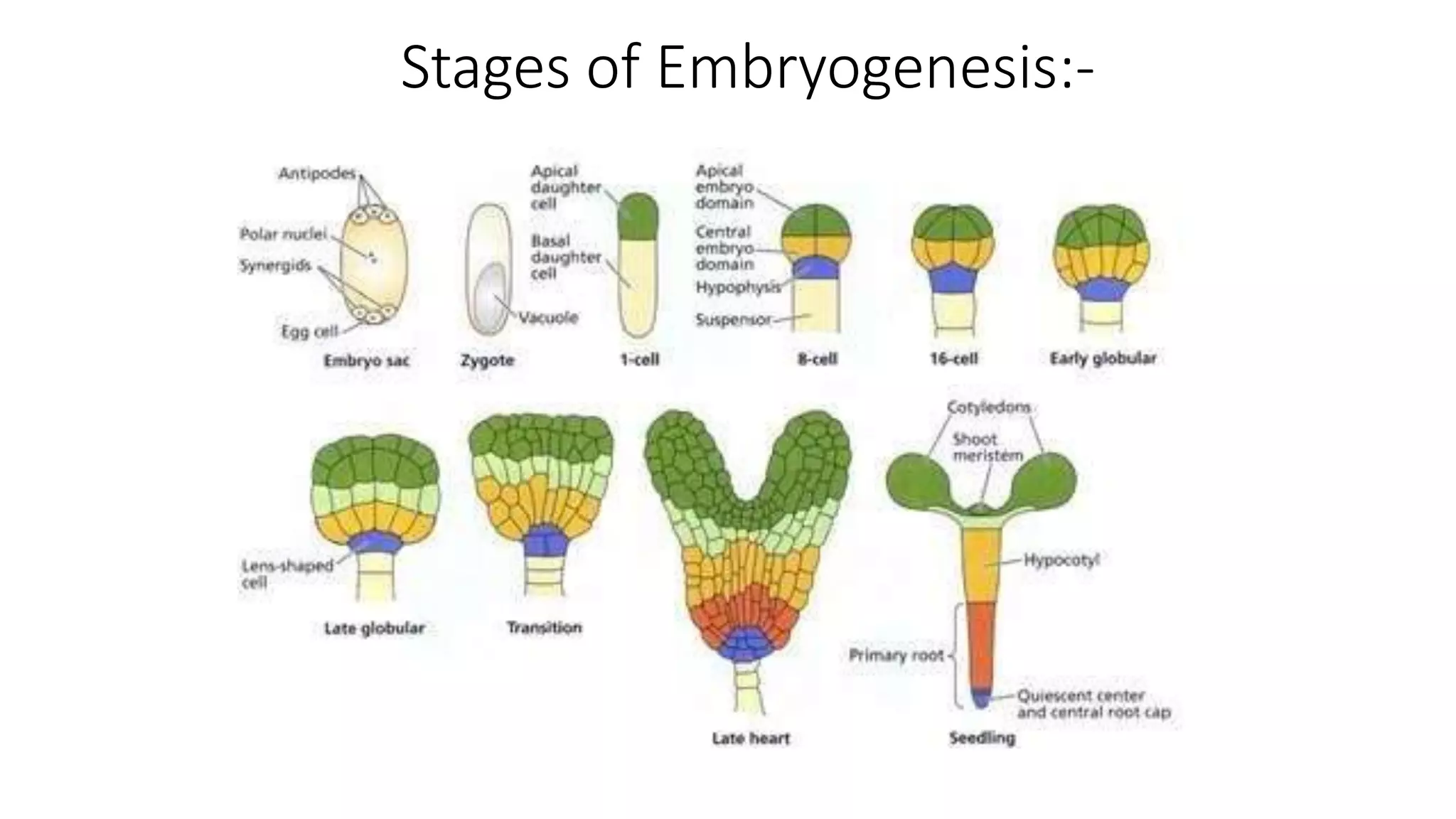
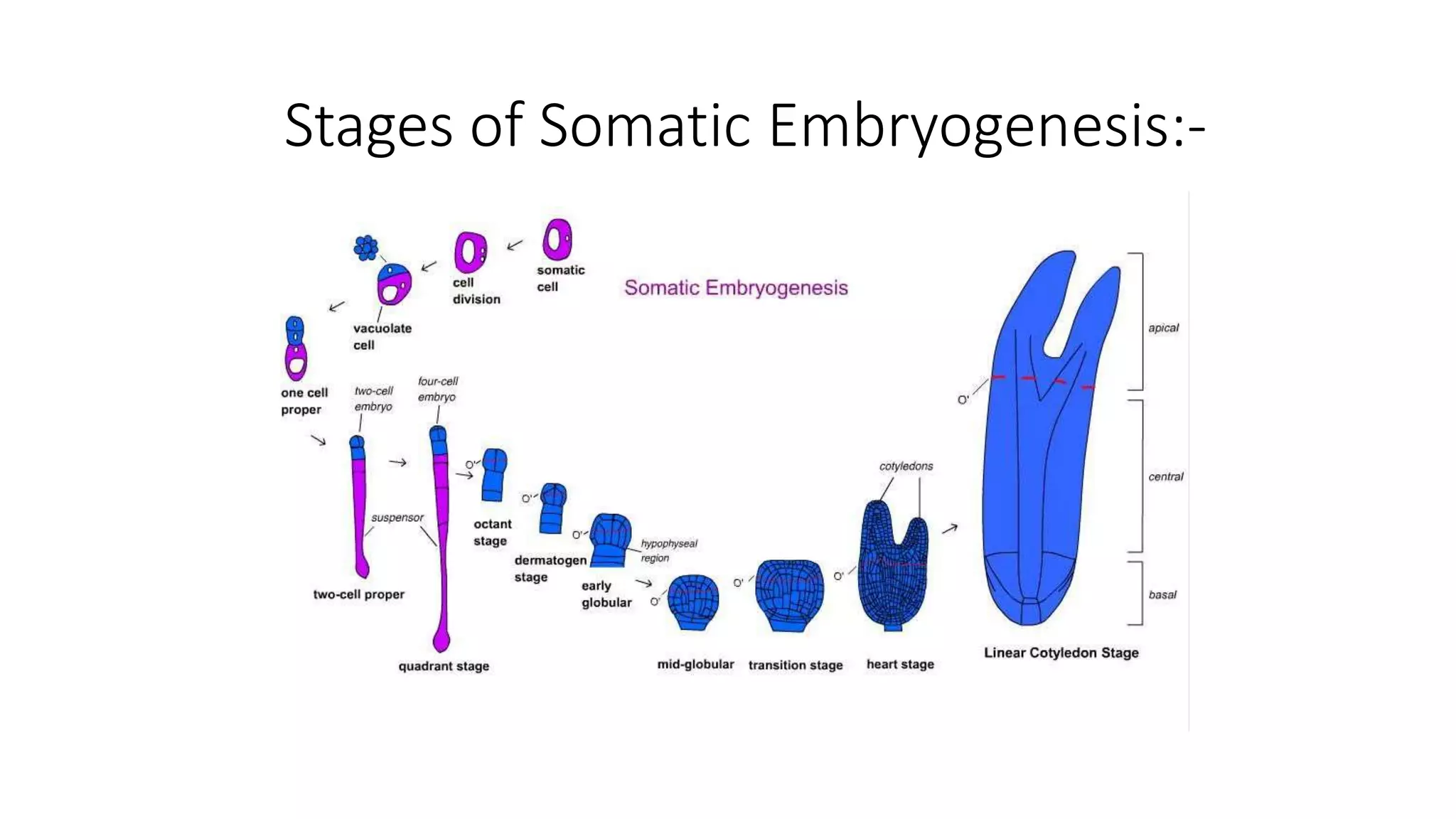
The document describes the process of somatic embryogenesis. It involves 7 key steps:
1) Induction of embryogenesis from explant tissue on media supplemented with auxin
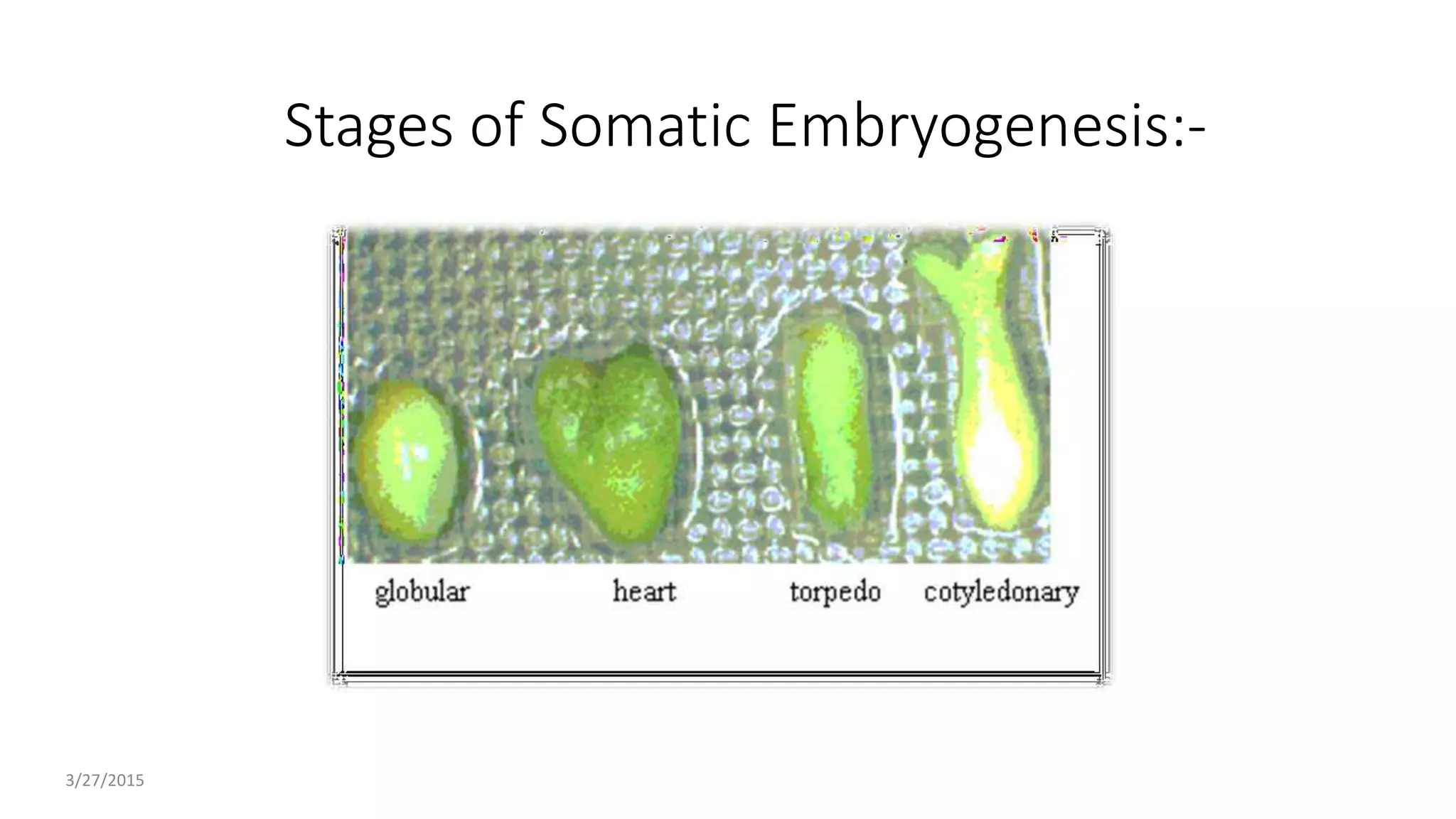
2) Development of somatic embryos through globular, heart, and torpedo stages of growth
3) Maturation of embryos with the formation of root and shoot meristems and cotyledons
4) Conversion of mature embryos to plantlets through germination on auxin-free media
Factors like explant type, growth regulators, and genotype influence the process. Somatic embryos differ from zygotic embryos in lacking a seed coat and having greater potential for propagation but weaker plantlets.































![8) Phytosulfokine
It modulate the culture media.
It promote somatic
embryogenesis by activating
cell division of embryogenic
cells, in presence auxin.
Phytosulfokine increases the
cell through differentiation
process.
9) Phenolic compounds:-
Phenolic compounds are inhibit
somatic embryogenesis.
4hydroxy benzyl alcohol inhibits
the globular stages.
Vanillyl benzyl ether are inhibit
the suspensor development.
Recently identification of 4
[(phenyl methoxy) methyl]
phenol involves in seed
development stills unknown.](https://image.slidesharecdn.com/morphogenesisorganogenesisembryogenesisothertechniques-210910113344/75/Morphogenesis-organogenesis-embryogenesis-amp-other-techniques-36-2048.jpg)































