Embed presentation
Downloaded 16 times
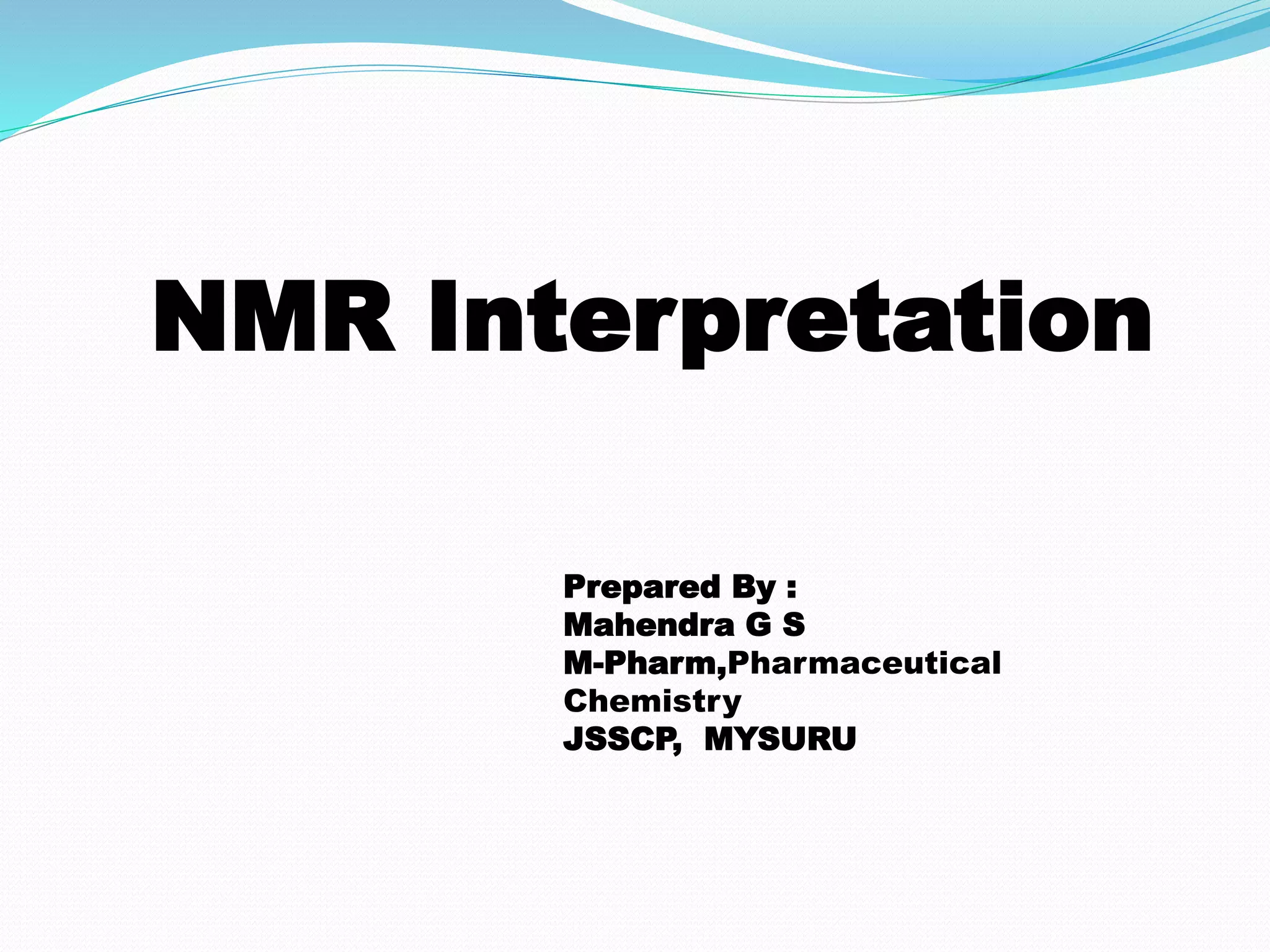

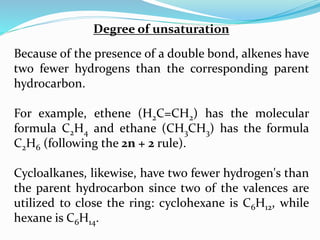
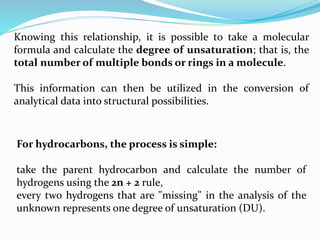
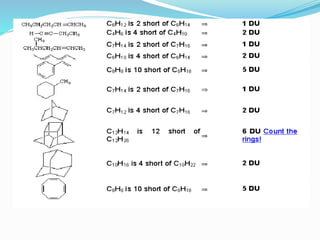

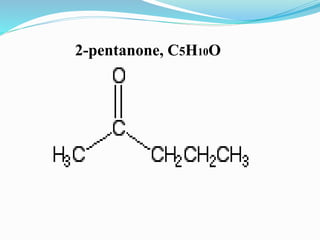
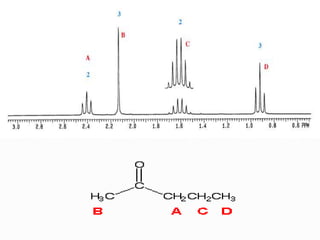
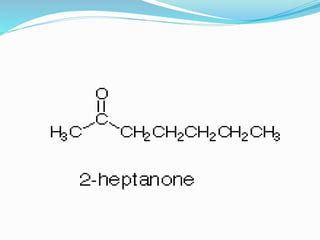
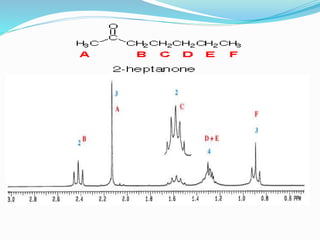
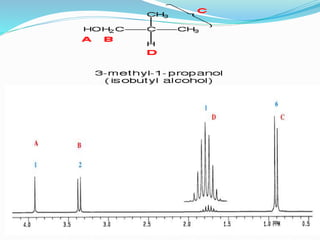
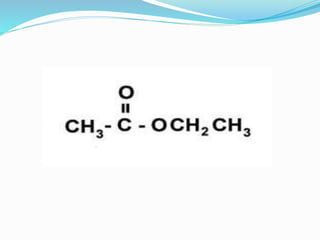
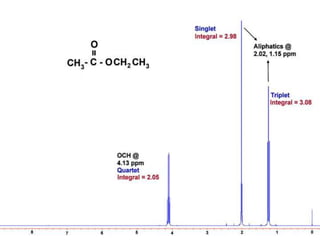
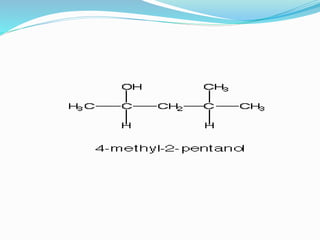
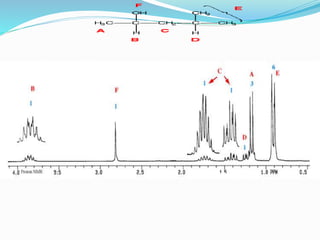
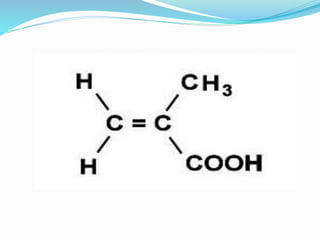
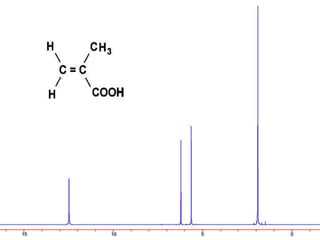
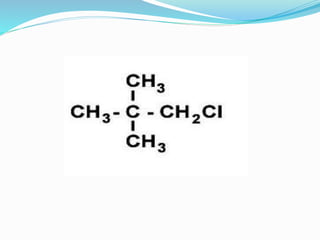
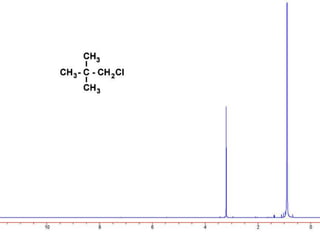
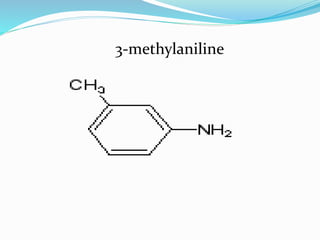
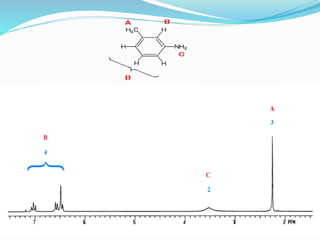
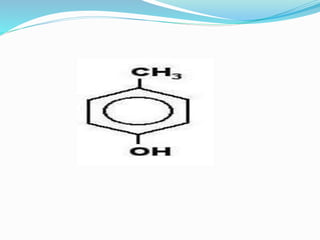
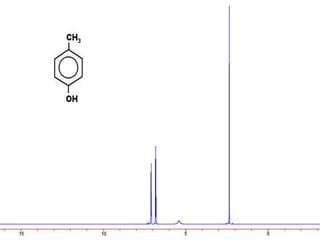
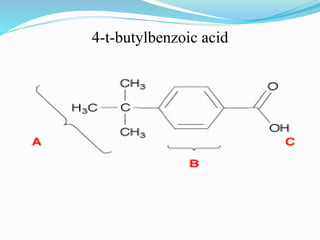
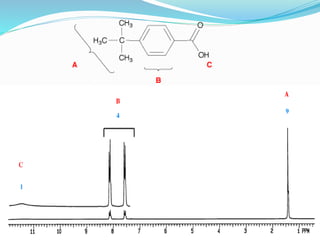
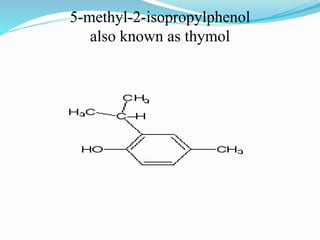
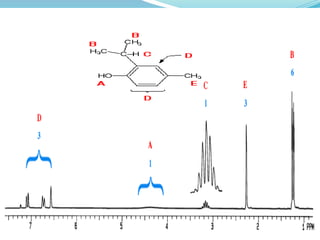
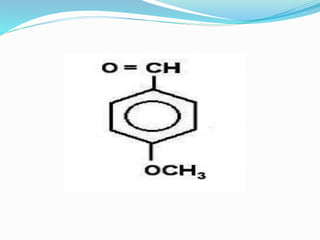
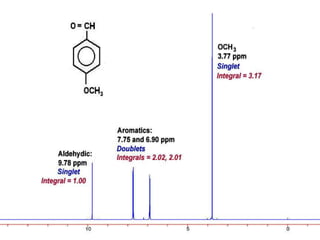
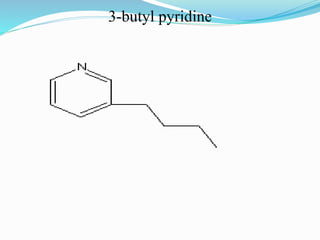
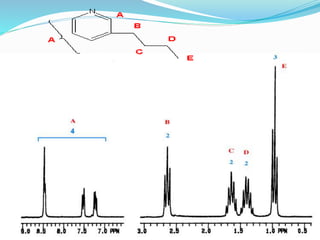

This document discusses NMR interpretation and provides examples of summarizing NMR data in 3 sentences or less: 1) It describes common NMR splitting patterns such as doublets, triplets, and multiplets based on the number of equivalent protons coupled. 2) It explains how to calculate the degree of unsaturation in a molecule from its molecular formula by determining the number of hydrogens missing based on the parent hydrocarbon. 3) Key aspects of NMR data to summarize are listed as the number of signals, their intensities, and splitting patterns.


































