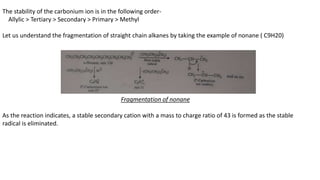
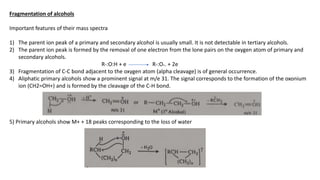
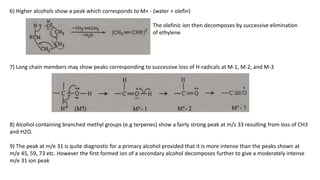
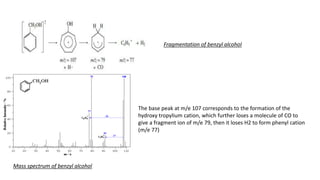
The document provides a detailed analysis of mass spectral fragmentation for various functional groups including alkanes, alcohols, aldehydes, ketones, and amines. It outlines the methodology for interpreting mass spectra, emphasizing the significance of molecular ions and fragmentation patterns specific to branched and straight-chain compounds. Key findings include the stability of fragment ions, the importance of cation stability in determining fragmentation, and characteristic peaks associated with specific functionalities.