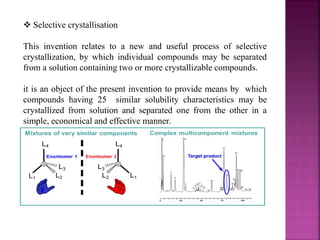
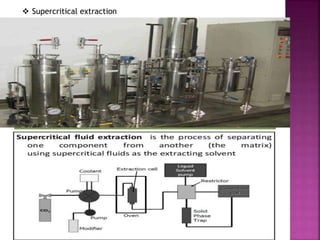
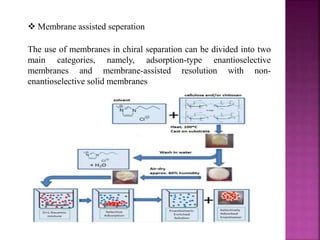
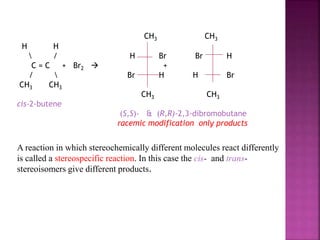
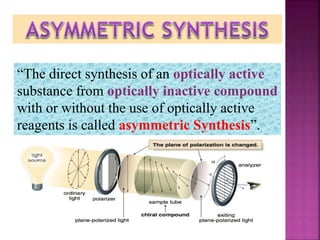
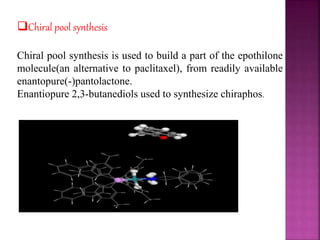
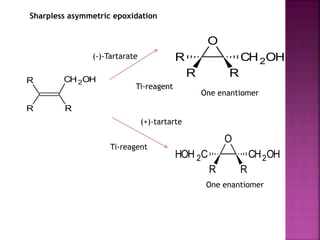
The document discusses asymmetric synthesis, focusing on methods such as chiral pool synthesis, chiral auxiliaries, and enantiopure separation techniques. It covers advancements in stereoselective synthesis and various catalysts used for selective reactions, as well as recent developments in the field. References to existing literature and studies are also provided to support the content.




![– Evans oxazolidinone auxiliaries are the most well known chiral
auxiliaries
• Derive from amino acids
- Forms the (Z)-‐boron enolates
– High stereoselectivity is attributed to the relatively short boron–oxygen
bond length
– Form a tight,six membered chair like transition state,Carbonyl is
opposed to the enolate oxygen dipole.
O N
O
CH3
O
Bn
CO2H
Ph
Me
OH
O NH
CH2
Bn
Bu2BOTf
O N
O O
Bn
Ph
OH
Me
LiOH,H2O2
Phcho
+
(4S)-4-benzyl-3-propanoyl-1,3-oxazolidin-2-one
(4S)-4-benzyl-3-[(2S,3S)-3-hydroxy-2-methyl-3-phenylpropanoyl]-1,3-oxazolidin-2-one
(4S)-4-benzyl-2-methylidene-1,3-oxazolidine
(a) (b)
(a)
(b)](https://image.slidesharecdn.com/finalasymmetricppt-200408181055/85/Asymmetric-synthesis-8-320.jpg)