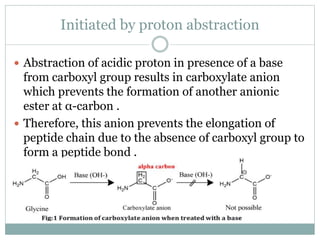
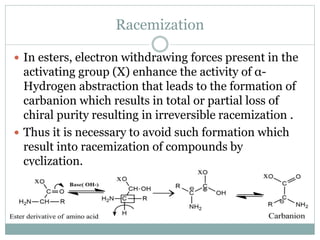
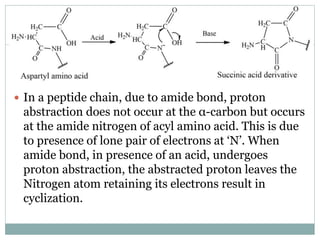
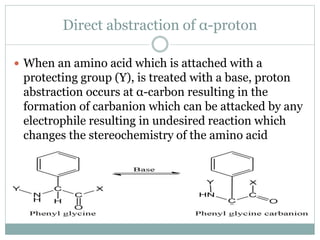
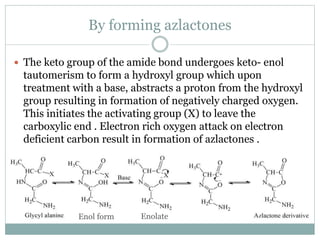
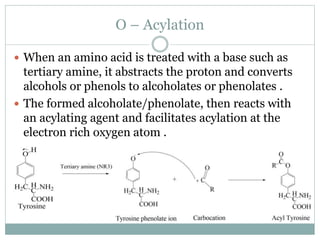
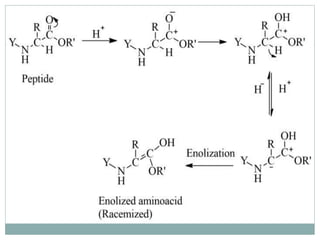
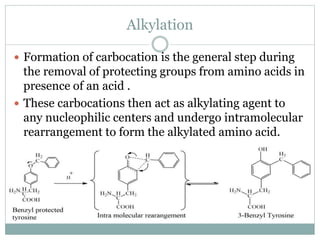
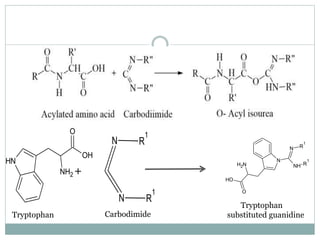
This document discusses common side reactions that can occur during peptide synthesis, including initiation by proton abstraction, protonation, and overactivation. Proton abstraction can lead to carboxylate anion formation or racemization. Protonation can cause racemization through carbocation formation or alkylation. Overactivation occurs when the carboxyl component is too reactive, leading to unwanted acylation of amino or hydroxyl groups. Understanding these side reactions is important for optimizing peptide synthesis techniques.