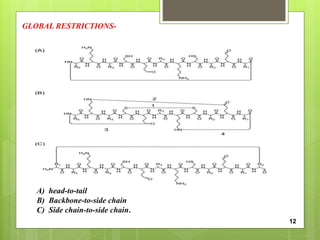
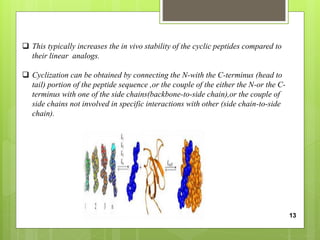
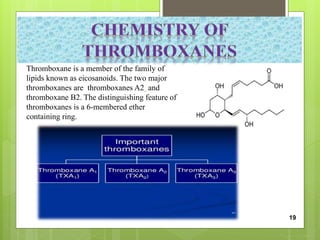
The document discusses peptidomimetics, which are designed to mimic peptides and can play a crucial role in cancer treatment by inducing apoptosis in cancer cells. It describes various types of peptidomimetics and their therapeutic values including anti-microbial, anti-malarial, anti-viral, and anti-cancer activities. Additionally, it delves into the structural properties of amino acids, peptide modifications for improved pharmacological properties, and discusses related molecules like prostaglandins, leukotrienes, and thromboxanes.