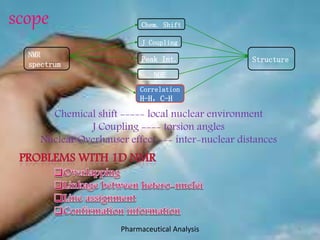
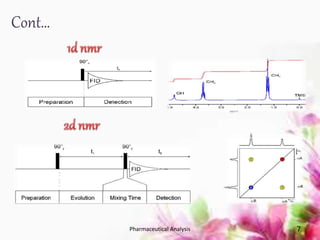
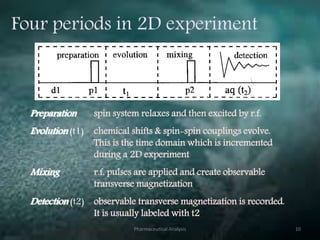
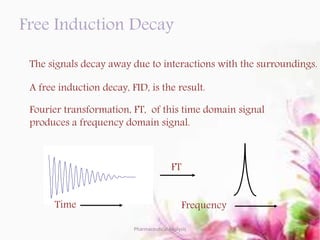
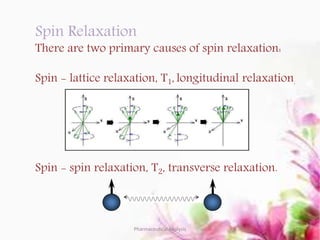
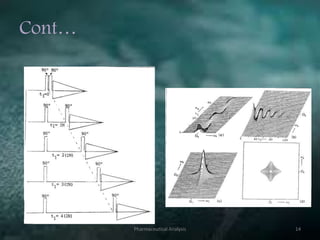
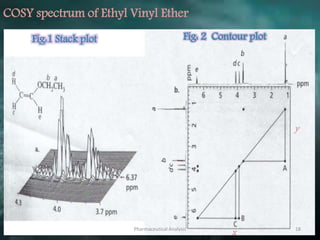
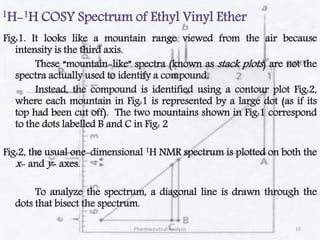
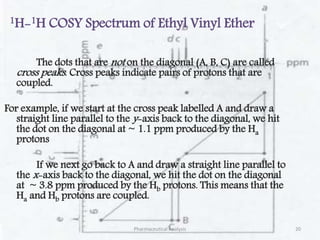
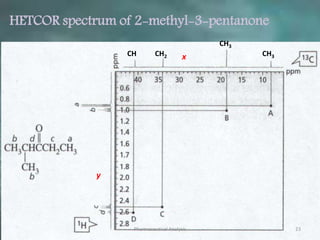
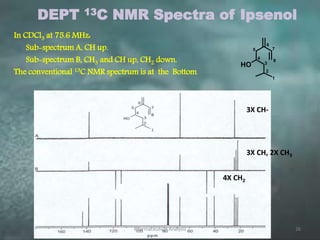
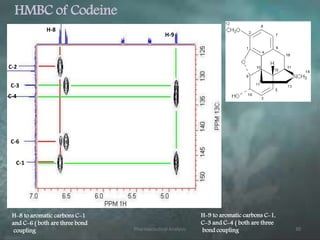
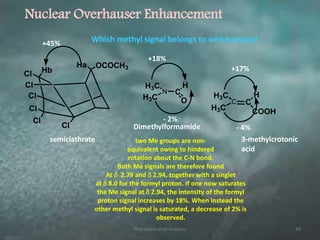
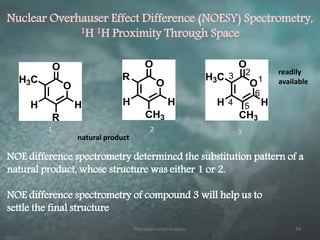
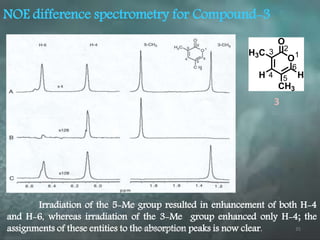
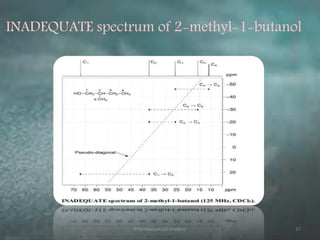
This document discusses various 2D NMR techniques used in pharmaceutical analysis including COSY, NOESY, HSQC, HMBC, and INADEQUATE. It explains the principles and applications of each technique. COSY identifies protons that are coupled through bonds, while NOESY identifies protons that are spatially close. HSQC and HMBC correlate 1H and 13C signals to determine connectivity. INADEQUATE directly shows 13C-13C connectivity but has low sensitivity. Together, these 2D NMR methods provide detailed structural information about pharmaceutical compounds.