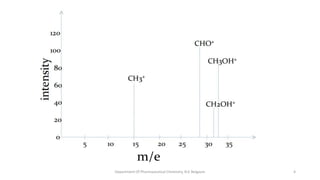
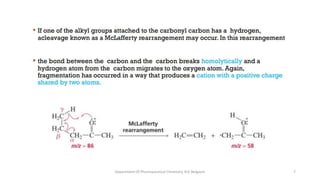
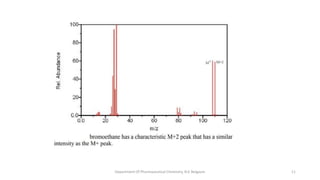
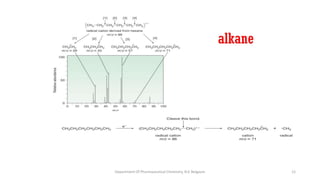
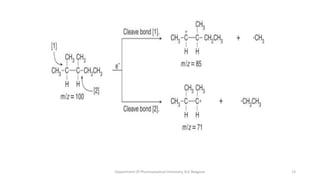
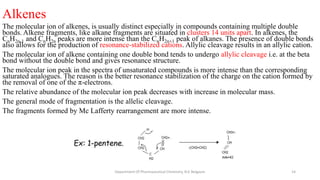
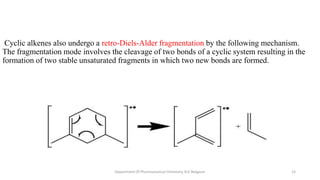
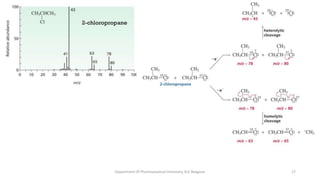
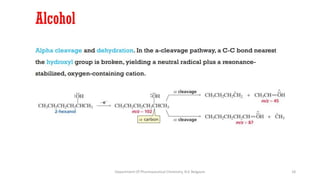
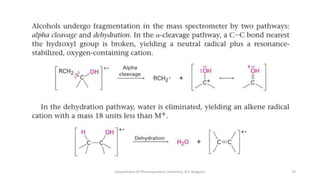
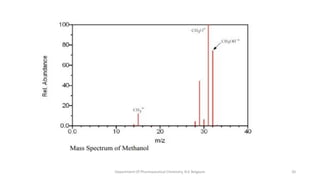
This document presents an overview of mass spectrometry. It discusses fragmentation rules, rearrangements like McLafferty rearrangement, the ring rule for calculating unsaturation, and isotopic peaks. It also examines the mass spectrometry of specific functional groups like alkanes, alkenes, alcohols, alkyl halides, and provides references for further reading. The presentation aims to cover basic concepts in mass spectrometry including how different functional groups fragment in the mass spectrometer.