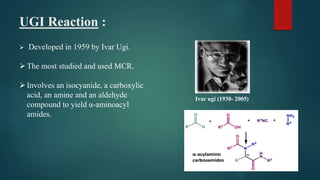
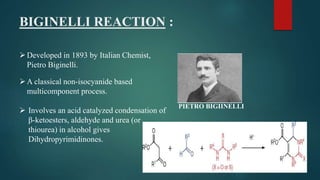
This document provides an overview of multi-component reactions (MCRs), including their history, advantages over multistep reactions, and examples such as the Passerini reaction, Ugi reaction, Biginelli reaction, and Mannich reaction. MCRs involve more than two starting materials reacting in one pot to form a product containing the majority of atoms from the reactants. They provide an efficient means of generating structural diversity and are important in drug discovery. Some of the earliest and most widely used MCRs are isocyanide-based reactions developed in the early 20th century.