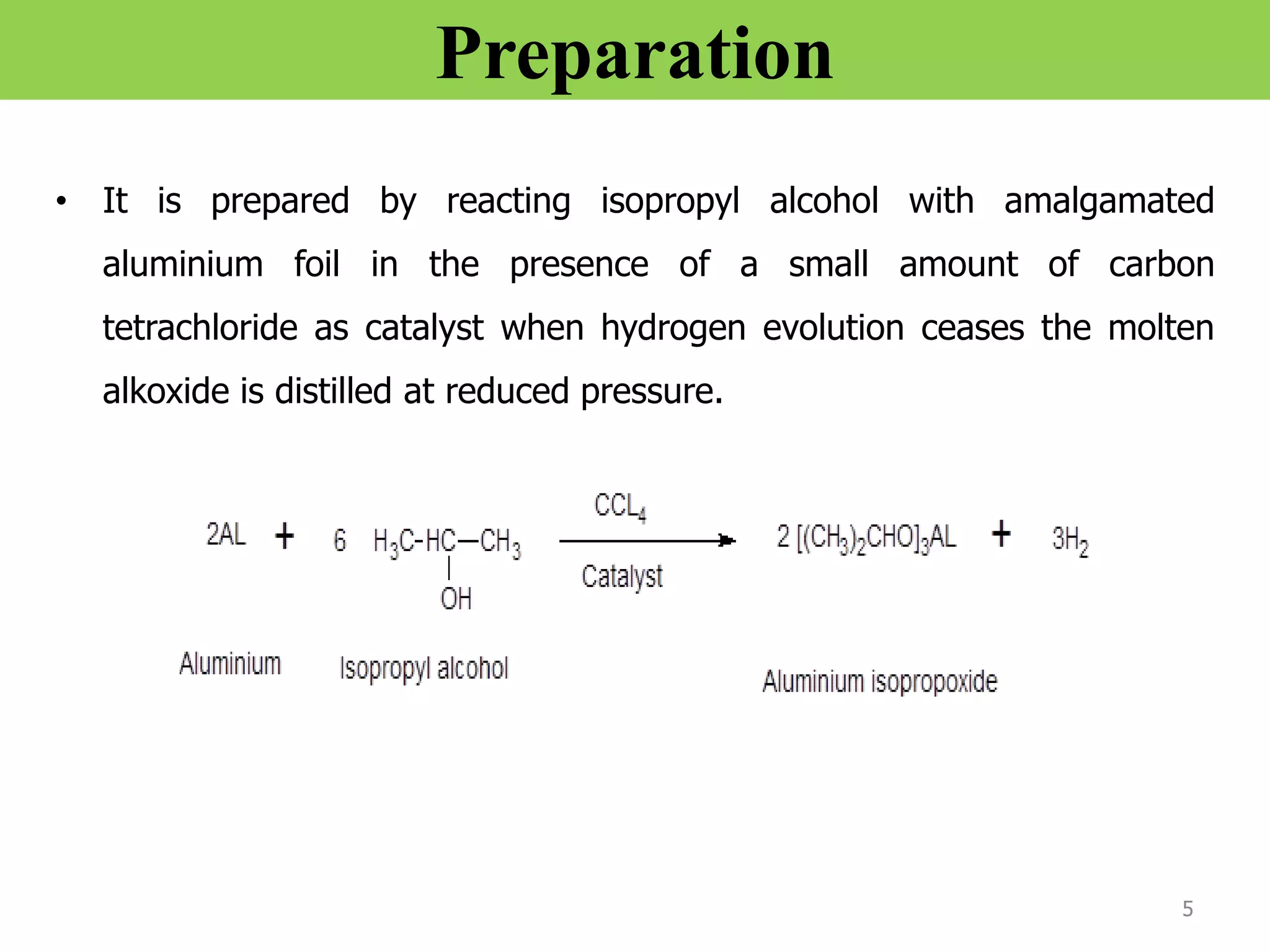
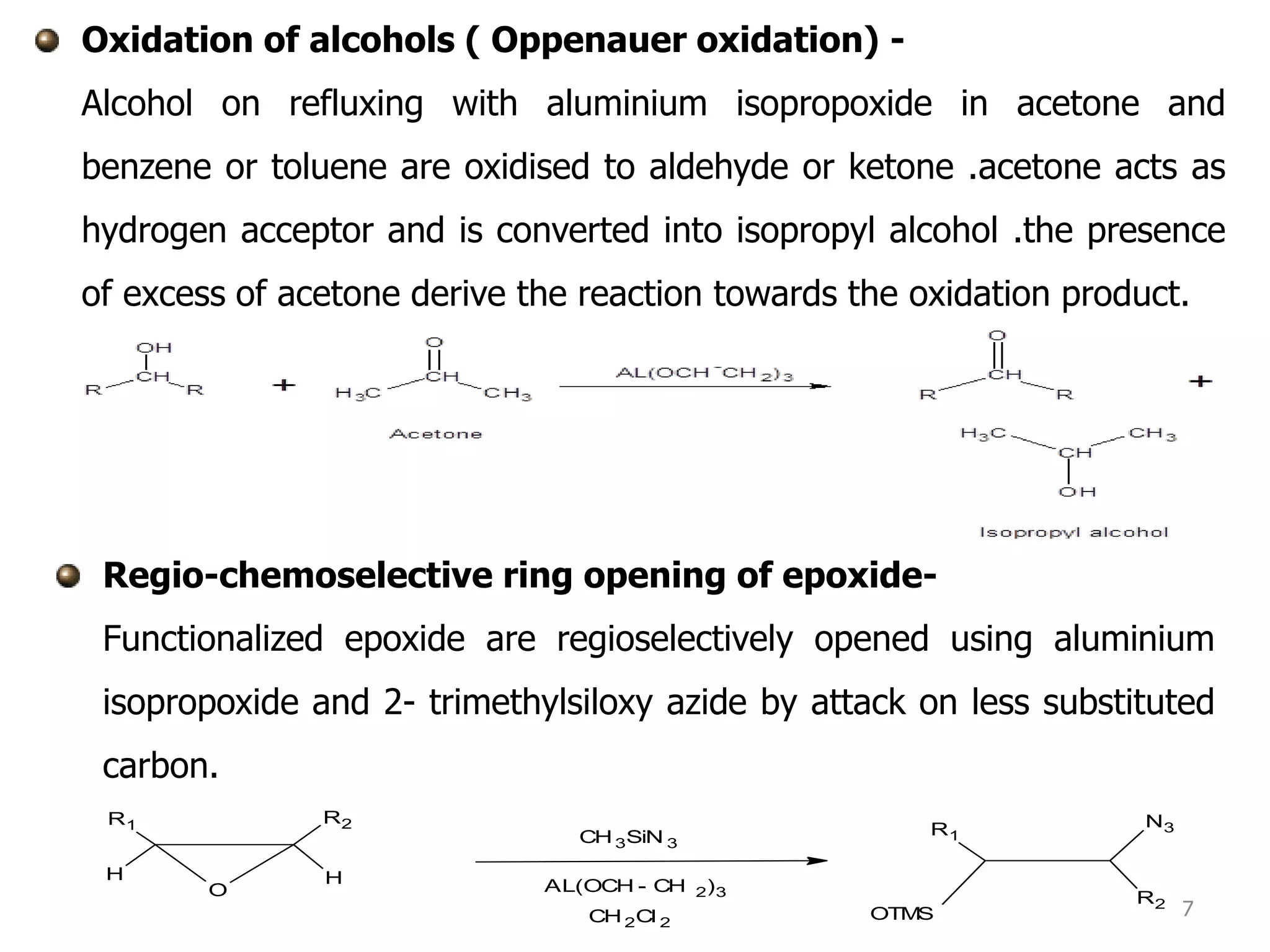
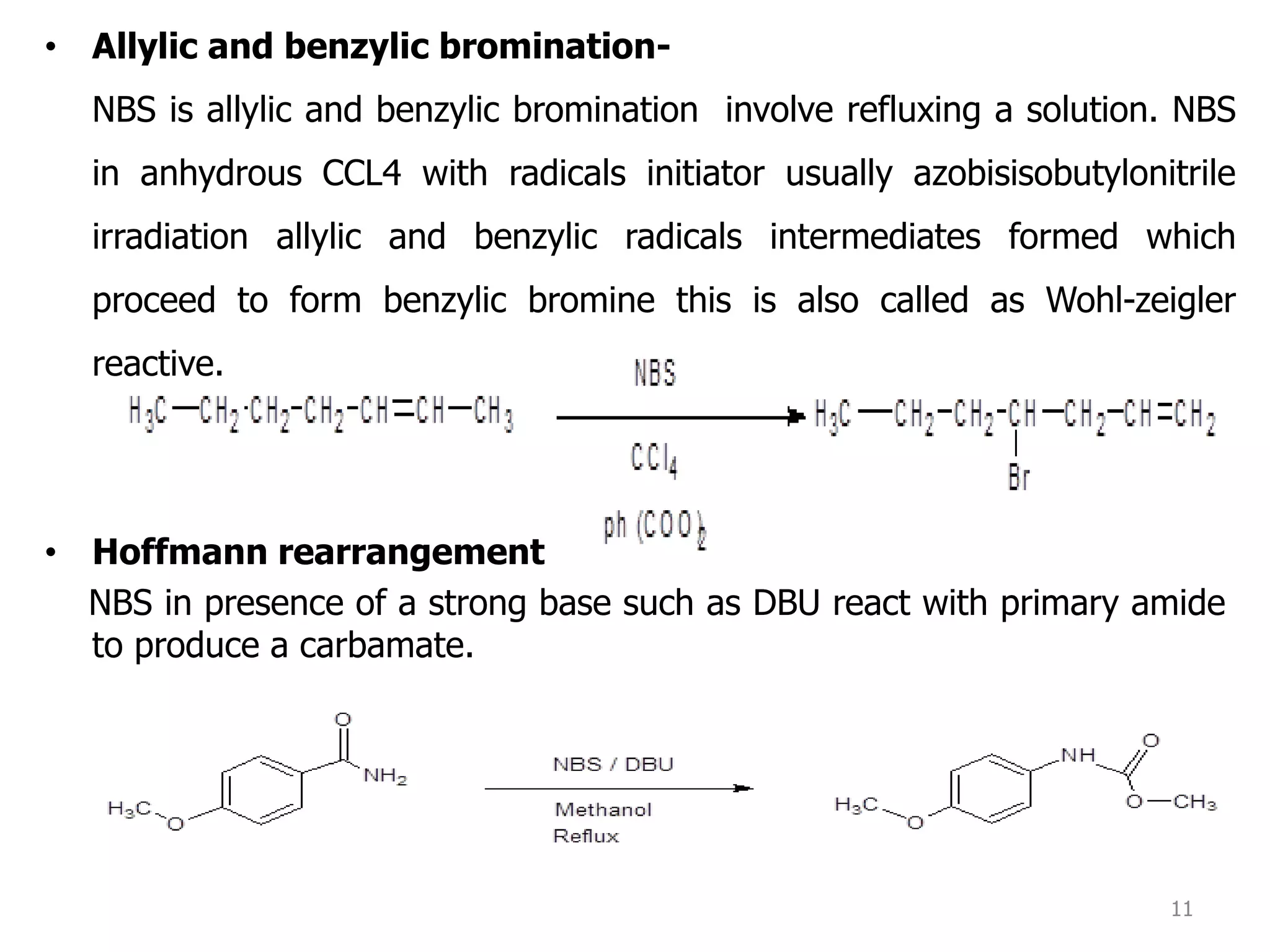
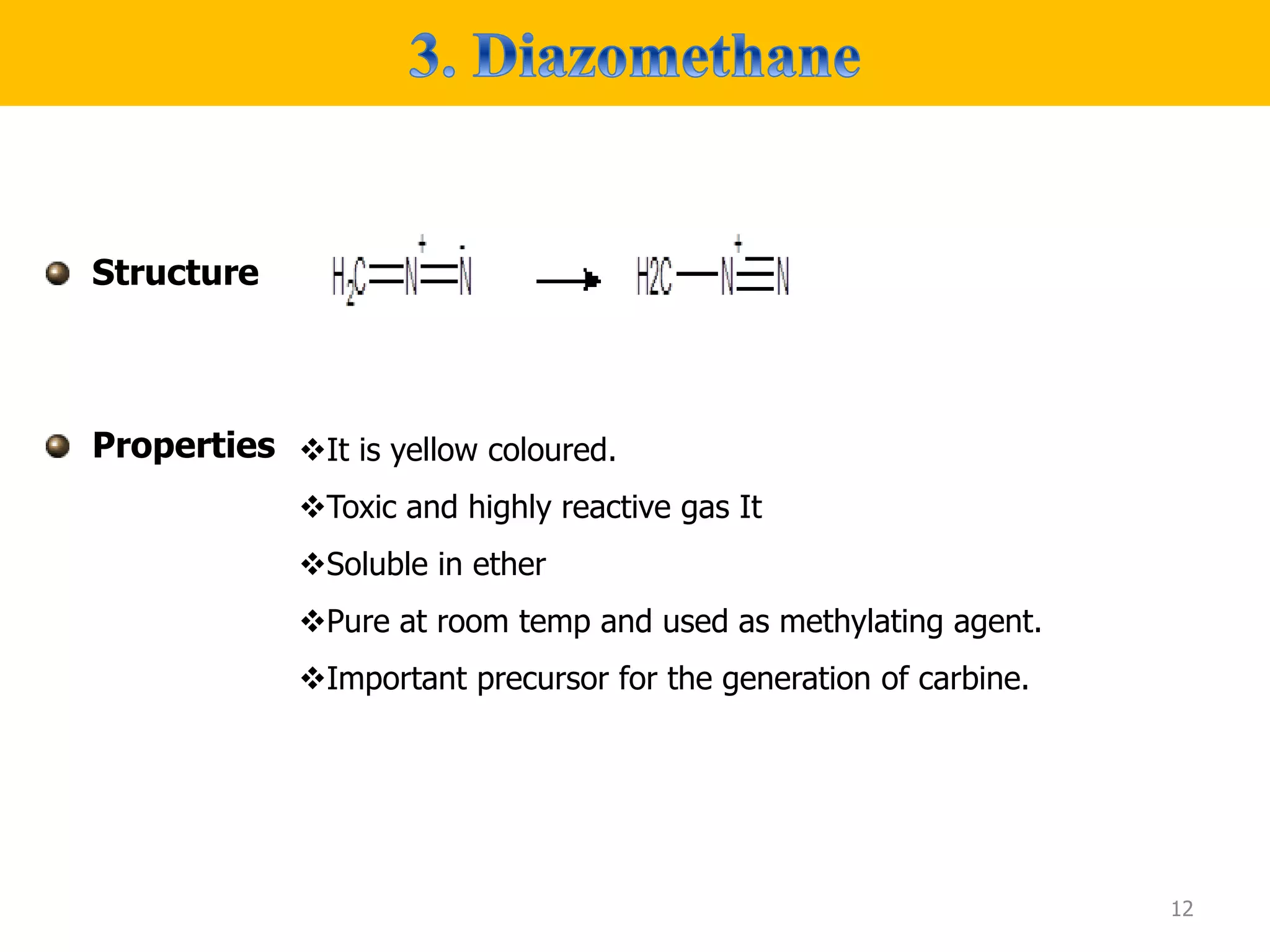
The document discusses various synthetic reagents used in pharmaceutical chemistry, including aluminium isopropoxide, n-bromo succinimide (NBS), diazomethane, and n,n-dicyclohexyl carbodiimide (DCC), highlighting their molecular properties, preparation methods, and applications in chemical reactions. Key uses include the oxidation of alcohols, hydrolysis of oximes, and synthesis of peptides. Additionally, it details the preparations and reactions associated with Wilkinson's catalyst and Witting reagent in alkene synthesis.













![Preparation
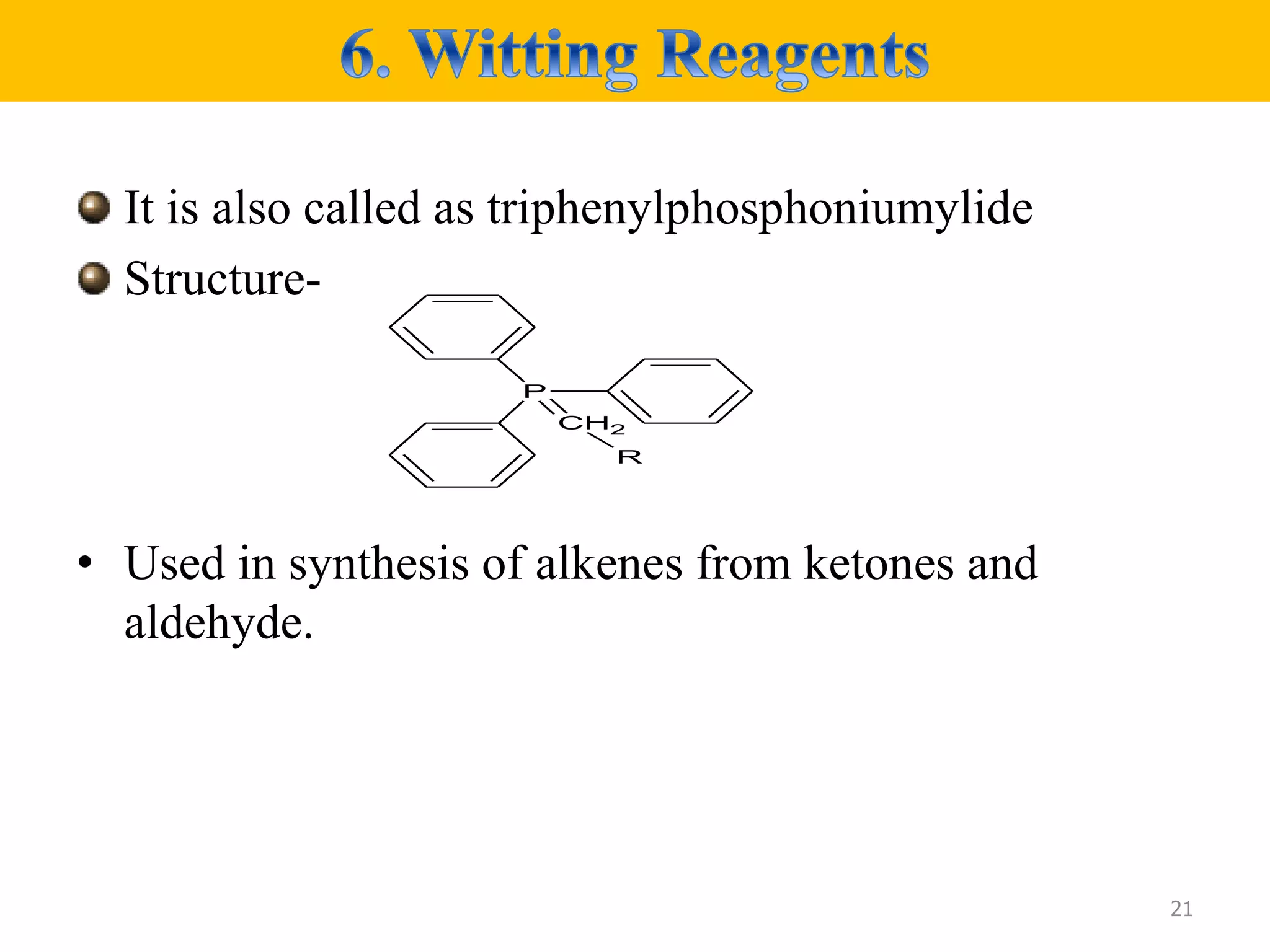
• Prepared from phosphonium salt which is in turn prepared by
quaternization of triphenylphosphine with an alkyl halide .the alkyl
phosphonium salt is deprotonated.
22
[PPh3CH2R] + C4H9 PPh3=CHR+ C4H10
Application
Synthesis of leucotrines
Witting reaction –It involve synthesis of alkenes from ketones
Prepration of olefins –
Aldehyde or ketones react with witting reagent it gives olefins.](https://image.slidesharecdn.com/ranislideshare-200225102955/75/Synthetic-Reagents-and-Its-Application-22-2048.jpg)
