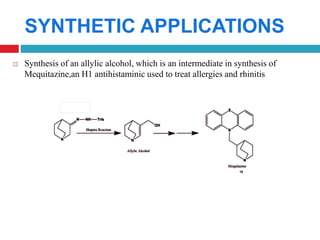
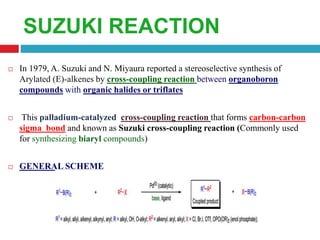
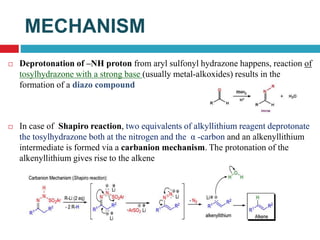
The document summarizes the Suzuki and Shapiro reactions. The Suzuki reaction involves a palladium-catalyzed cross-coupling between organoboron compounds and organic halides to form carbon-carbon bonds. It proceeds through oxidative addition, transmetallation, and reductive elimination steps. The Shapiro reaction involves the base-catalyzed decomposition of tosyl hydrazones to form olefins. Both reactions have been used in the synthesis of various drugs and natural products.



![MECHANISM
STEP1 – OXIDATIVE ADDITION - The rate determining step of the catalytic
cycle in which an organic halide couples to the Palladium catalyt to form
organopalladium complex[Pd(0)-species to form Pd(II); as two groups add to the
metal]
STEP2 – Exchange of the anion attached to the palladium for the anion of the
base; Hydroxide attacks Pd, kicking off X](https://image.slidesharecdn.com/suxukiandshapiroreaction-211208102459/85/Suzuki-and-Shapiro-reaction-5-320.jpg)

![ STEP 4 – Reductive elimination- The final step that forms the C-C sigma
bond and also regenerates the Palladium catalyst[ Pd(0) ] so that it can
participate again in catalytic cycle
The order of reductive elimination is as follows : Ar-Ar > Ar-R > R-R](https://image.slidesharecdn.com/suxukiandshapiroreaction-211208102459/85/Suzuki-and-Shapiro-reaction-7-320.jpg)











![ Bamford Steven Reaction mechanism
In aprotic condition, initially formed diazo compound loses a molecule of
nitrogen and forms a carbene intermediate, that undergoes a [1,2]-H shift and
gives rise to alkene](https://image.slidesharecdn.com/suxukiandshapiroreaction-211208102459/85/Suzuki-and-Shapiro-reaction-20-320.jpg)