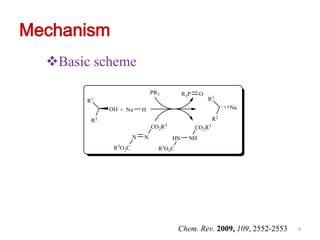
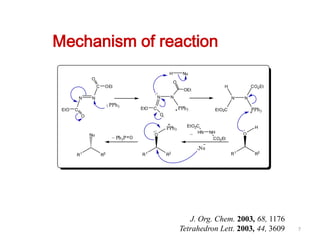
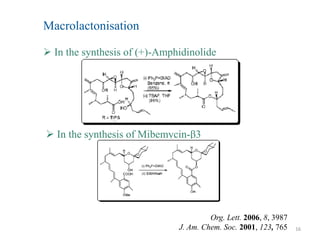
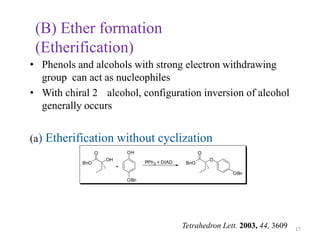
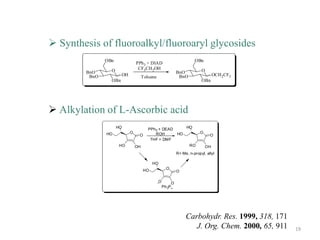
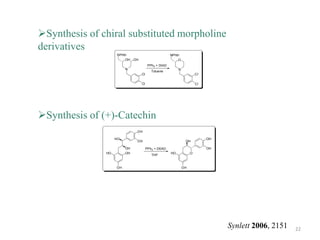
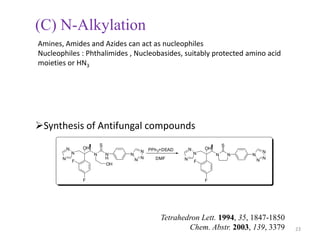
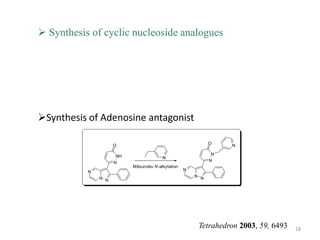
The Mitsunobu reaction allows the conversion of alcohols to various functional groups using trialkyl/triaryl phosphine and dialkyl azodicarboxylate reagents. It proceeds via an oxidation-reduction mechanism. Common applications include esterification, etherification, and N-alkylation reactions. Recent advances have focused on replacing conventional reagents to improve selectivity and yields. The Mitsunobu reaction has been widely used in the synthesis of natural products and pharmaceuticals.