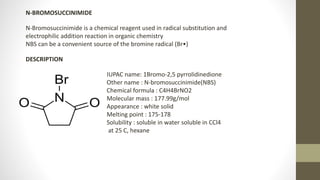
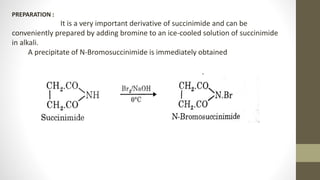
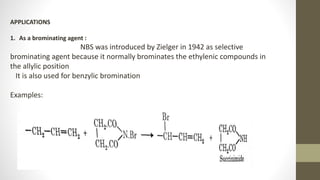
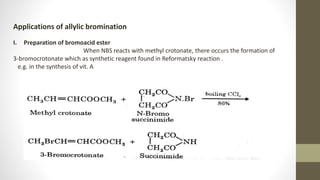
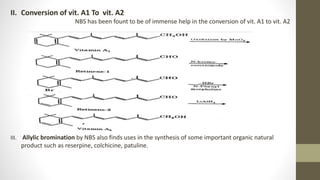
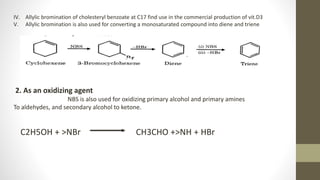
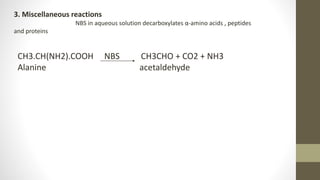
N-Bromosuccinimide (NBS) is a chemical reagent used for radical substitution and electrophilic addition reactions. It can be conveniently prepared by adding bromine to an ice-cooled solution of succinimide in alkali. NBS is commonly used as a brominating agent, selectively adding bromine to allylic positions. It acts as a bromine reservoir, maintaining a lower concentration of molecular bromine. NBS can also oxidize primary alcohols and amines to aldehydes and ketones.