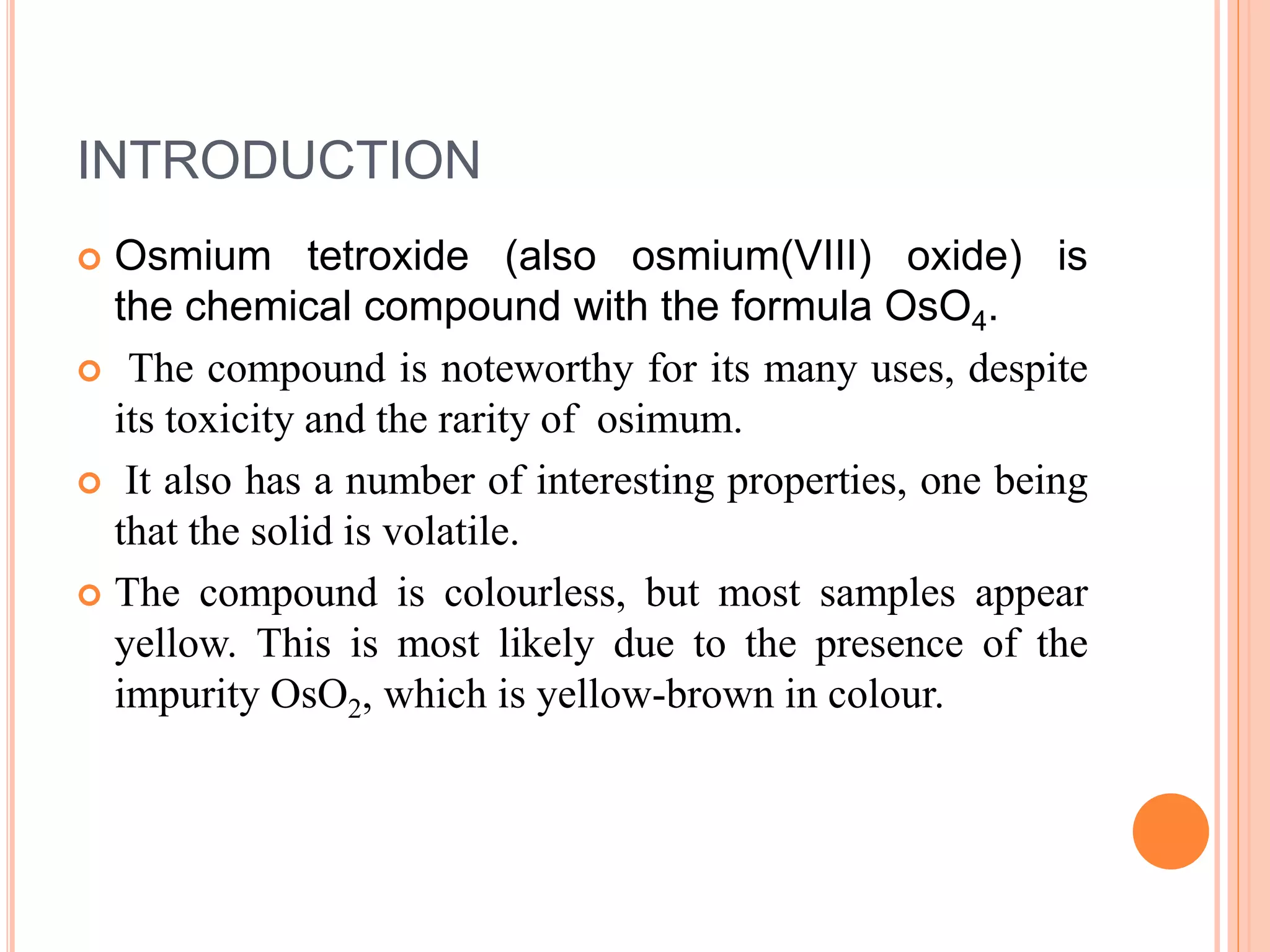
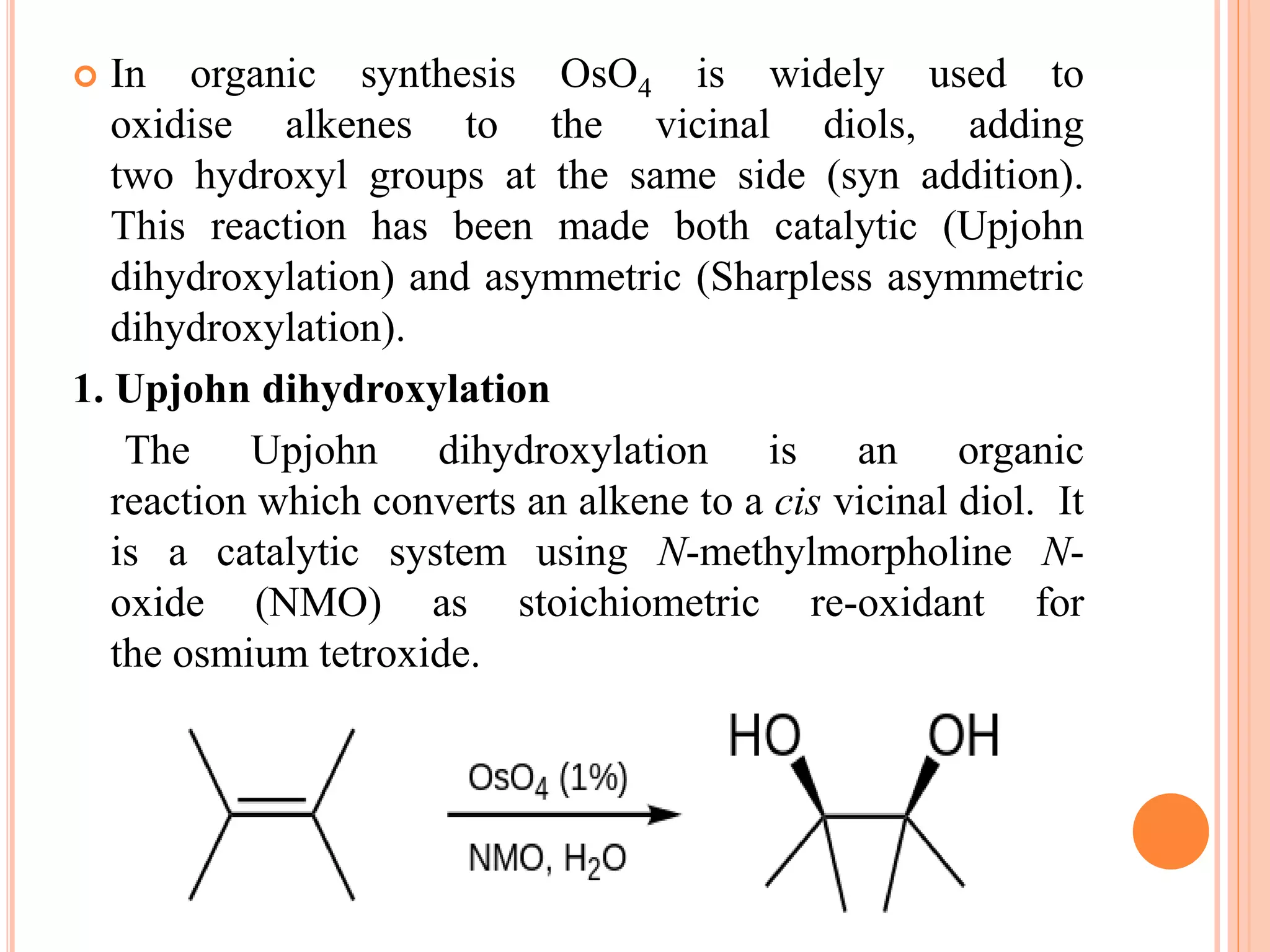
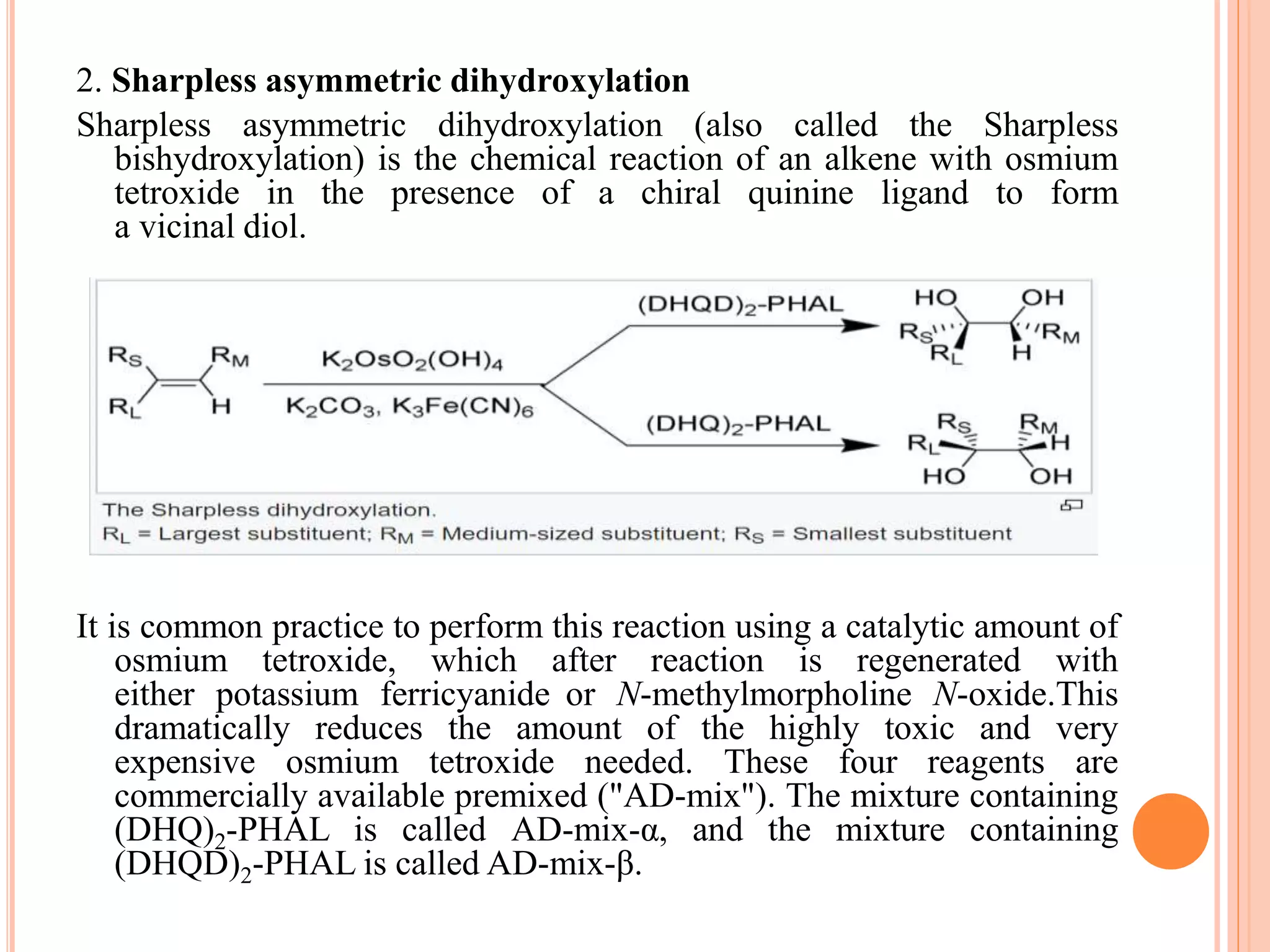
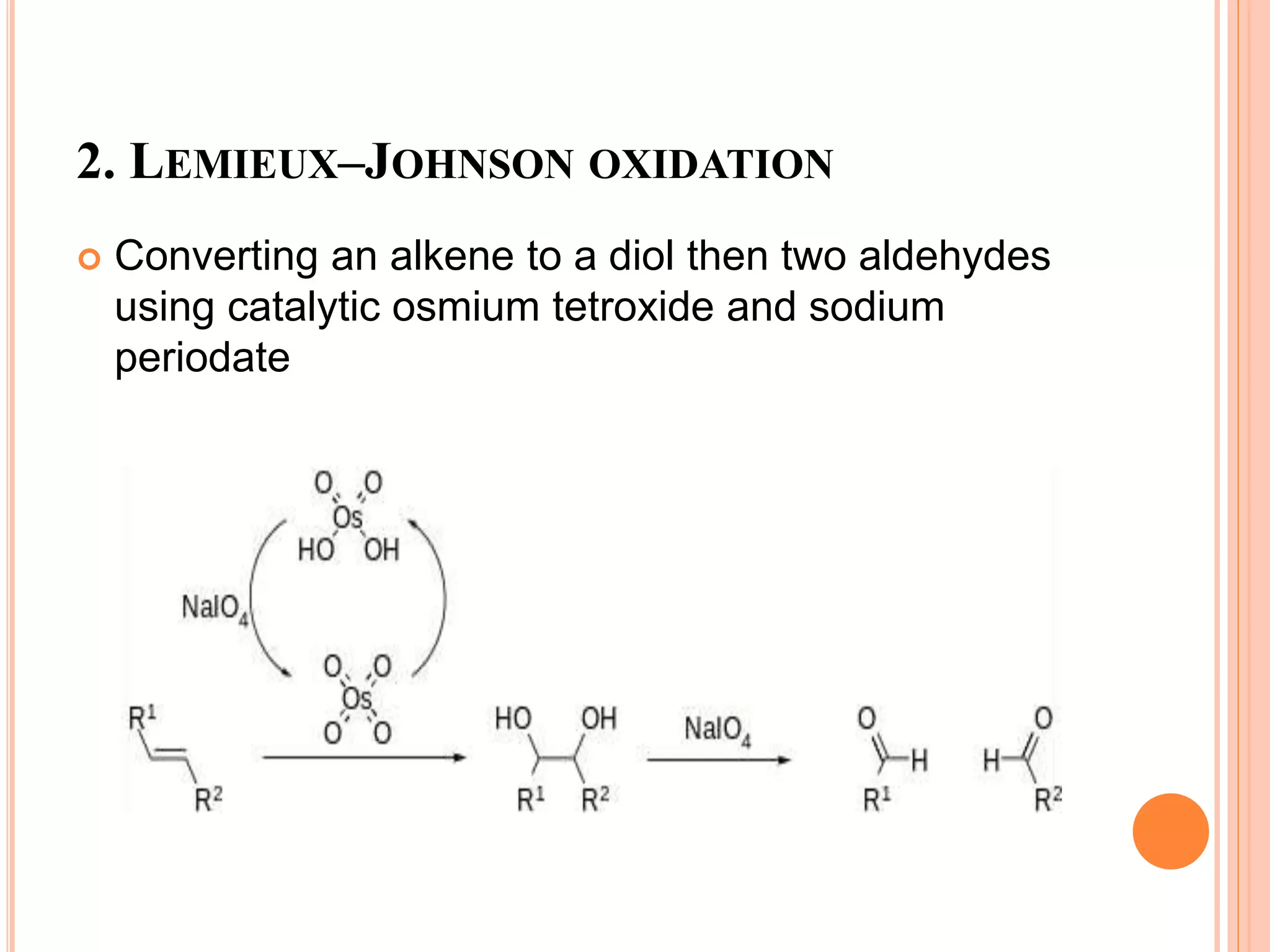
Osmium tetroxide (OsO4) is a volatile, colorless compound with a chlorine-like odor. It is noteworthy for its many uses in organic synthesis despite its toxicity. OsO4 is widely used to oxidize alkenes to vicinal diols through a [3+2] cycloaddition reaction. It is also used in the Lemieux-Johnson oxidation to convert an alkene to a diol and then two aldehydes. Additionally, OsO4 forms complexes with amines and fluorides and can be reduced to osmium metal.



![APPLICATION OF OSMIUM TETROXIDE
I. Oxidation of alkenes
Alkenes add to OsO4 to give diolate species that hydrolyze
to cis-diols. The net process is called dihydroxylation. This
proceeds via a [3+ 2] cycloaddition reaction between the
OsO4 and alkene to form an intermediate osmate ester which
rapidly hydrolyses to yield the vicinal diol. As the oxygen
atoms are added in a concerted step the resulting
stereochemistry is cis](https://image.slidesharecdn.com/osmiumtetroxide-181124050303/75/Osmium-tetroxide-8-2048.jpg)

![3. COORDINATION CHEMISTRY
When the Lewis base is an amine, adducts are also
formed. Thus OsO4 can be stored in the form of osmeth,
in which OsO4 is complexed with hexamine
1. With tert-BuNH2, the imido derivative is produced:
OsO4 + Me3CNH2 → OsO3(NCMe3) + H2O
2. With NH3 one obtains the nitrido complex:
OsO4 + NH3 + KOH → K[Os(N)O3] + 2 H2O](https://image.slidesharecdn.com/osmiumtetroxide-181124050303/75/Osmium-tetroxide-13-2048.jpg)

![4. OXOFLUORIDES
Osmium forms several oxofluorides, all of which are
very sensitive to moisture. Purple cis-OsO2F4 forms at
77 K in an anhydrous KrF2 solution:
OsO4 + 2 KrF2 → cis-OsO2F4 + 2 Kr + O2
OsO4 also reacts with F2 to form yellowOsO3F2:
2 OsO4 + 2 F2 → 2 OsO3F2 + O2
OsO4 + 2 [Me4N]F → [Me4N]2[cis-OsO4F2]](https://image.slidesharecdn.com/osmiumtetroxide-181124050303/75/Osmium-tetroxide-15-2048.jpg)
