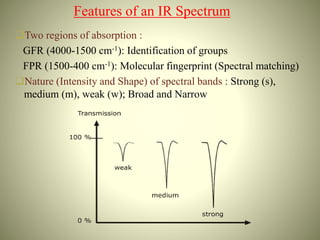
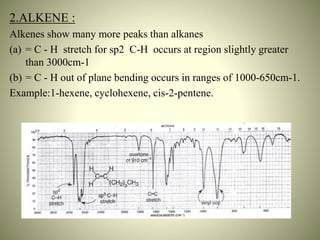
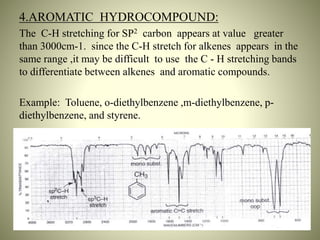
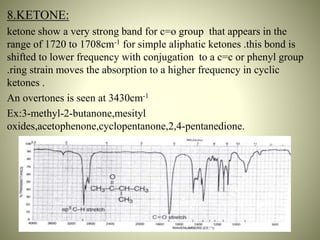
This document summarizes the infrared spectral features of various organic compounds. It discusses the absorption regions and functional groups of hydrocarbons such as alkanes, alkenes, alkynes and aromatics. It also covers alcohols, ethers, aldehydes, ketones, carboxylic acids, esters, amides, and amines. For each functional group, it provides examples and describes the characteristic absorption bands that can be used for identification in infrared spectroscopy. In conclusion, it notes that infrared spectroscopy provides detailed information about functional groups and molecular structure.