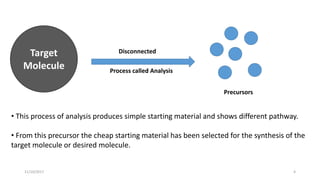
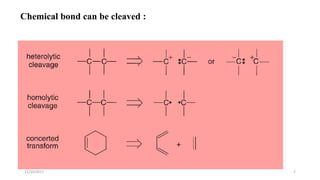
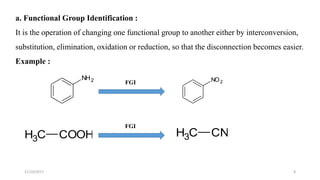
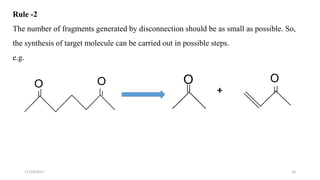
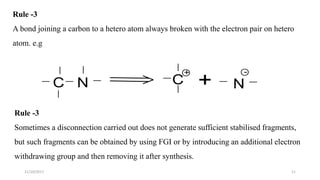
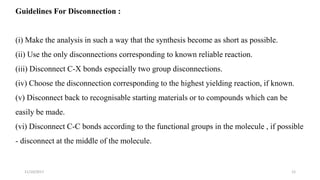
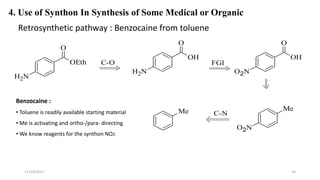
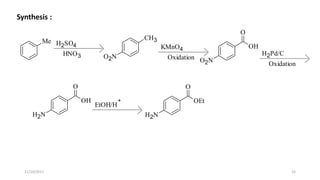
This document discusses the synthon approach to retrosynthetic analysis in organic synthesis. It defines key terms like disconnection, synthon, and functional group interconversion. The document outlines basic rules for disconnection, such as generating stable fragments and minimizing the number of fragments. It provides guidelines for good retrosynthesis and discusses the use of synthons in the multi-step synthesis of benzocaine from toluene as an example.