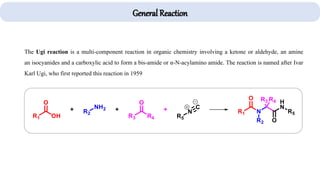
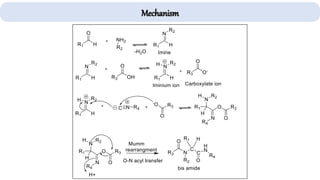
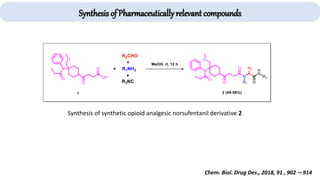
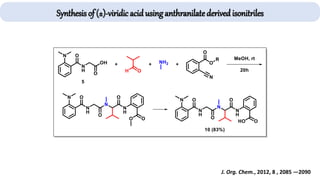
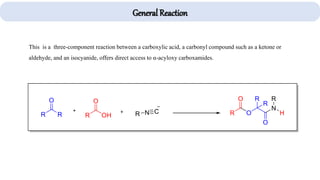
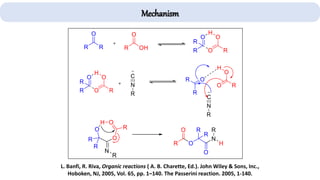
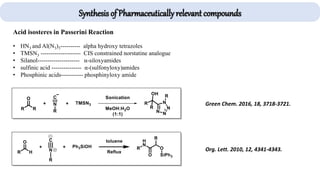
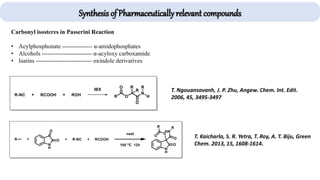
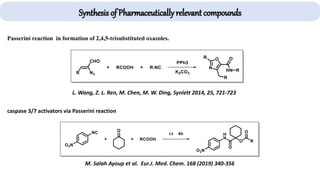
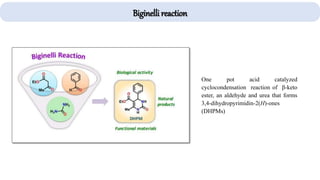
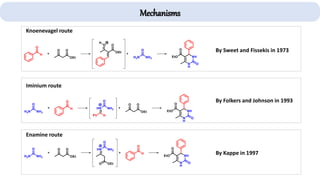
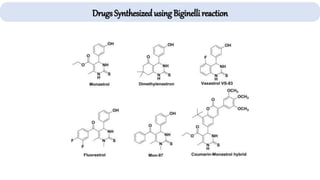
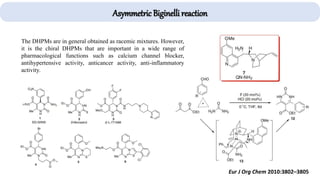
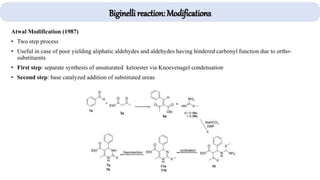
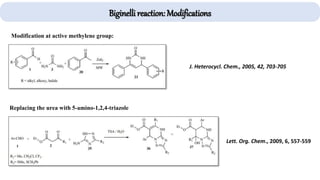
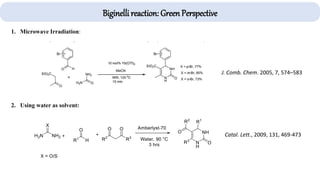
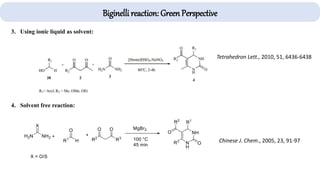
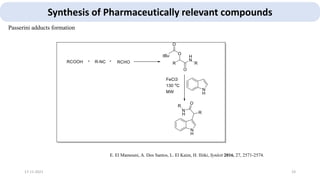
The document describes key multicomponent reactions in organic chemistry, including the Ugi, Passerini, and Biginelli reactions, highlighting their mechanisms and applications in synthesizing pharmaceutical compounds. Each reaction involves specific reactants and leads to the formation of various important intermediates or products, with some pathways showing modifications for improved yields and green chemistry perspectives. The significance of these reactions extends to their applications in drug synthesis and pharmacological functions.