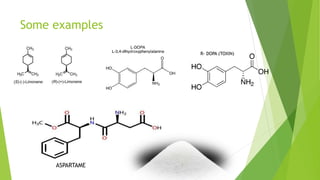
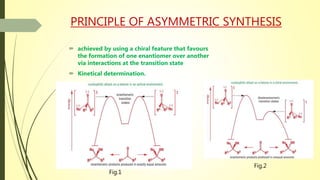
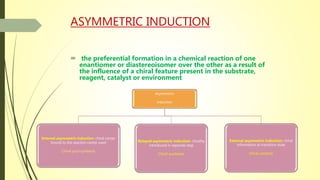
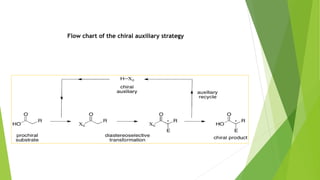
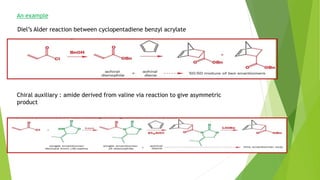
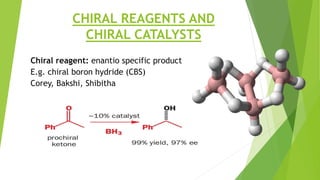
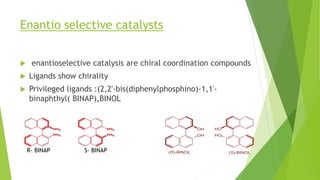
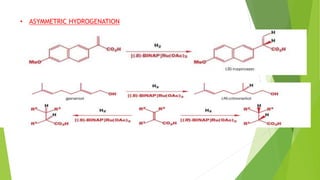
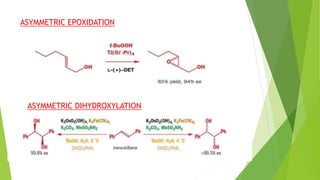
Asymmetric synthesis is a chemical reaction that produces one stereoisomer in greater amounts than the other. It is achieved through the use of a chiral feature like a substrate, reagent, catalyst, or environment that favors the formation of one enantiomer over the other in the transition state. Some common approaches for asymmetric synthesis include using a chiral starting material from nature, attaching a chiral auxiliary, or employing a chiral reagent or catalyst. The separation and analysis of enantiomers can be challenging given their identical physical properties, requiring techniques like chiral chromatography or crystallization. Asymmetric synthesis has important applications in pharmaceuticals for producing drugs that are safer and more effective.