- Macrolides are a class of antibiotics that are produced by Streptomyces bacteria and contain a macrocyclic lactone ring. Erythromycin was the first macrolide discovered in 1952.
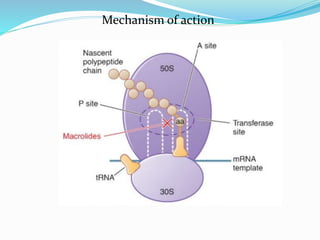
- Macrolides work by attaching to the 50S subunit of bacterial ribosomes and inhibiting protein synthesis. They are bacteriostatic and have selectivity for bacterial over mammalian cells.
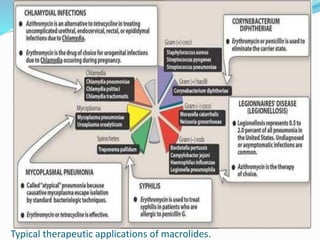
- Common macrolides include erythromycin, clarithromycin, roxithromycin, and azithromycin. They are effective against many gram-positive bacteria and some gram-negatives. Azithromycin has the broadest spectrum of activity.