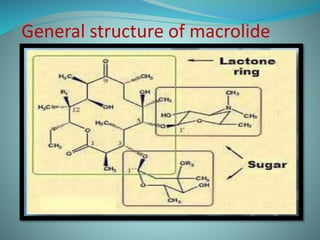
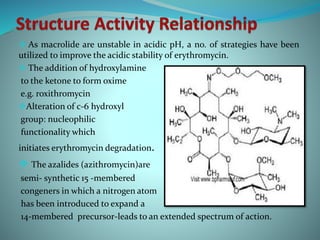
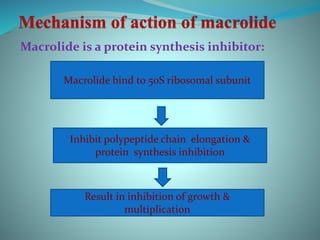
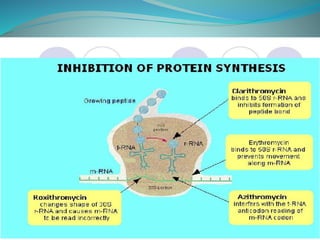
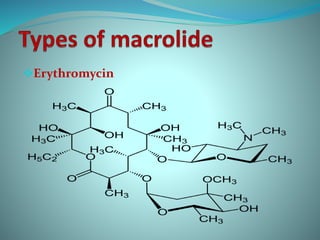
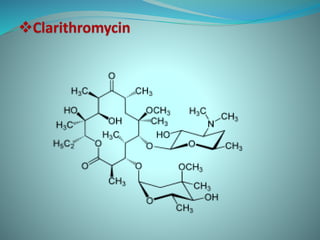
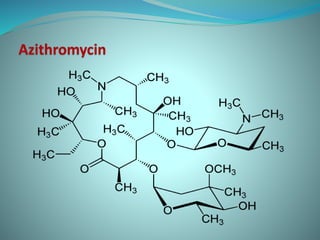
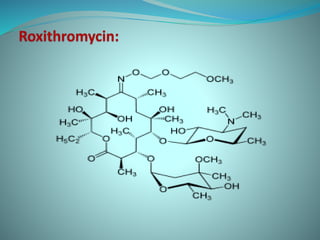
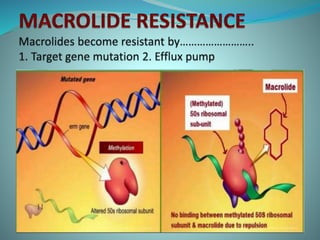
The document provides an in-depth overview of macrolide antibiotics, including their definition, chemical structure, mechanism of action, and types. It discusses their broad-spectrum activity against various infections, pharmacokinetics, resistance, indications, contraindications, and side effects. Specific macrolides like erythromycin, azithromycin, and clarithromycin are highlighted, along with their physical properties, dosage forms, and therapeutic uses.