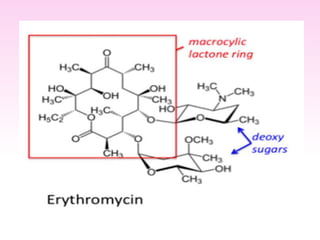
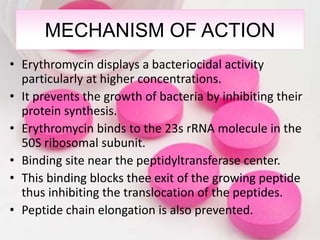
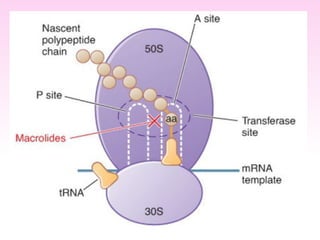
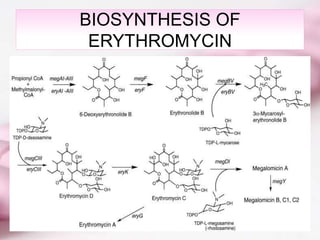
Erythromycin is a macrolide antibiotic discovered in 1952 that inhibits bacterial protein synthesis and growth. It is produced through fermentation of Streptomyces bacteria and extracted for use as an alternative to penicillin in individuals with penicillin allergies. Erythromycin contains a macrocyclic lactone ring with sugars attached and has bacteriostatic or bactericidal effects depending on concentration. It is commonly used to treat respiratory, genital, and eye infections caused by bacteria and chlamydia when penicillin cannot be used.