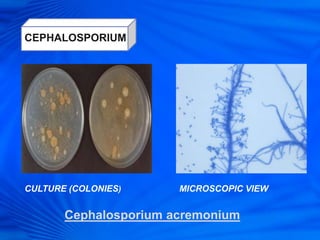
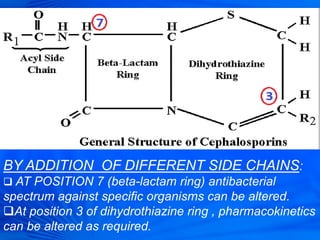
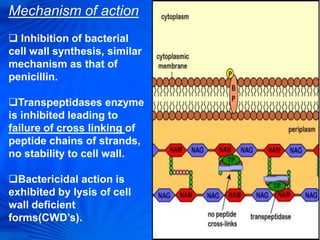
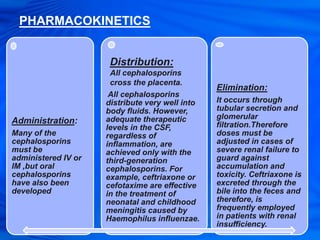
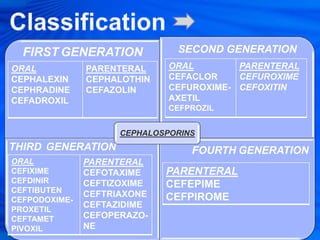
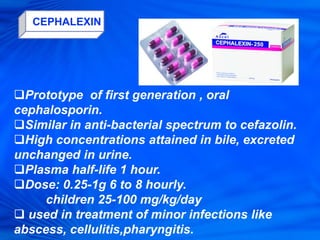
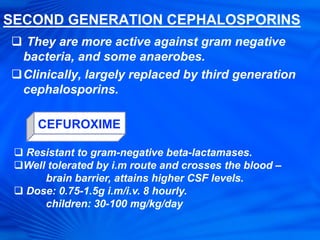
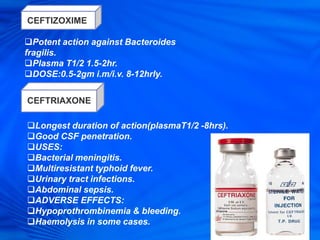
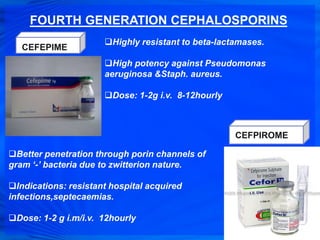
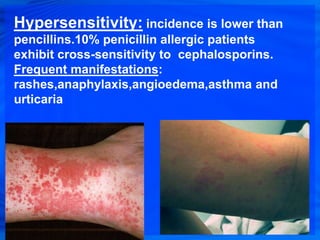
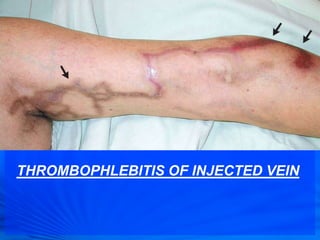
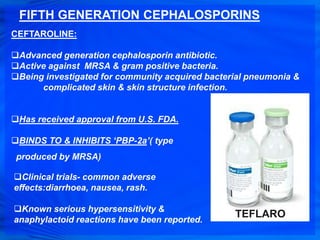
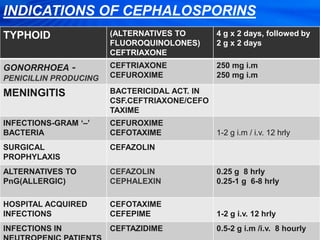
Cephalosporins are a class of antibiotics derived from the fungus Cephalosporium acremonium. They were first isolated in 1948 and are chemically related to penicillins. There are several generations of cephalosporins that have been developed with expanded spectra of activity. First generation cephalosporins such as cefazolin and cephalexin are effective against gram-positive bacteria. Later generations have activity against more gram-negative bacteria with third generation drugs like cefotaxime and ceftriaxone used to treat serious infections. Cephalosporins are generally well-tolerated but can cause adverse effects like diarrhea, rash, bleeding and hypersensitivity reactions in some