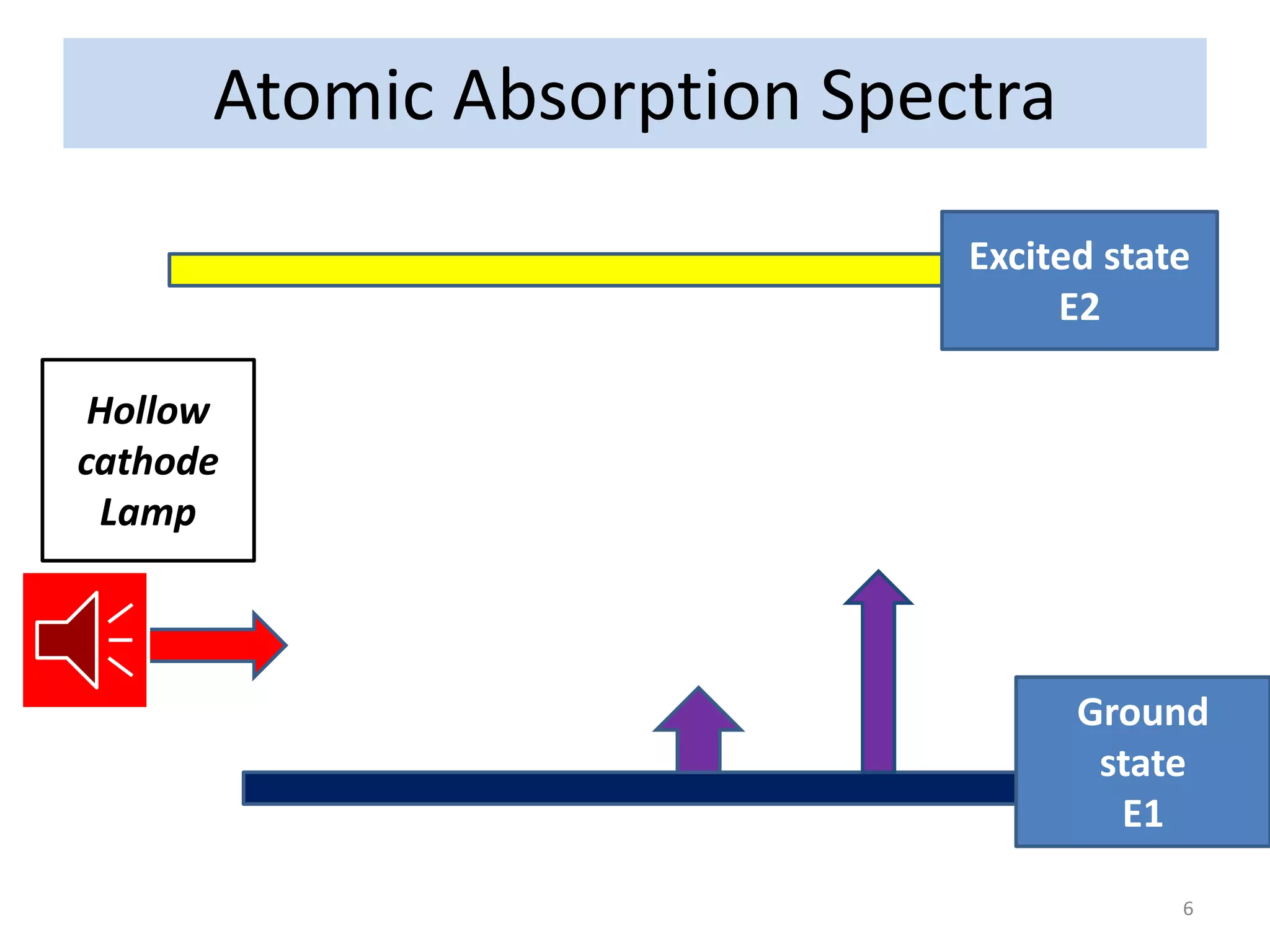
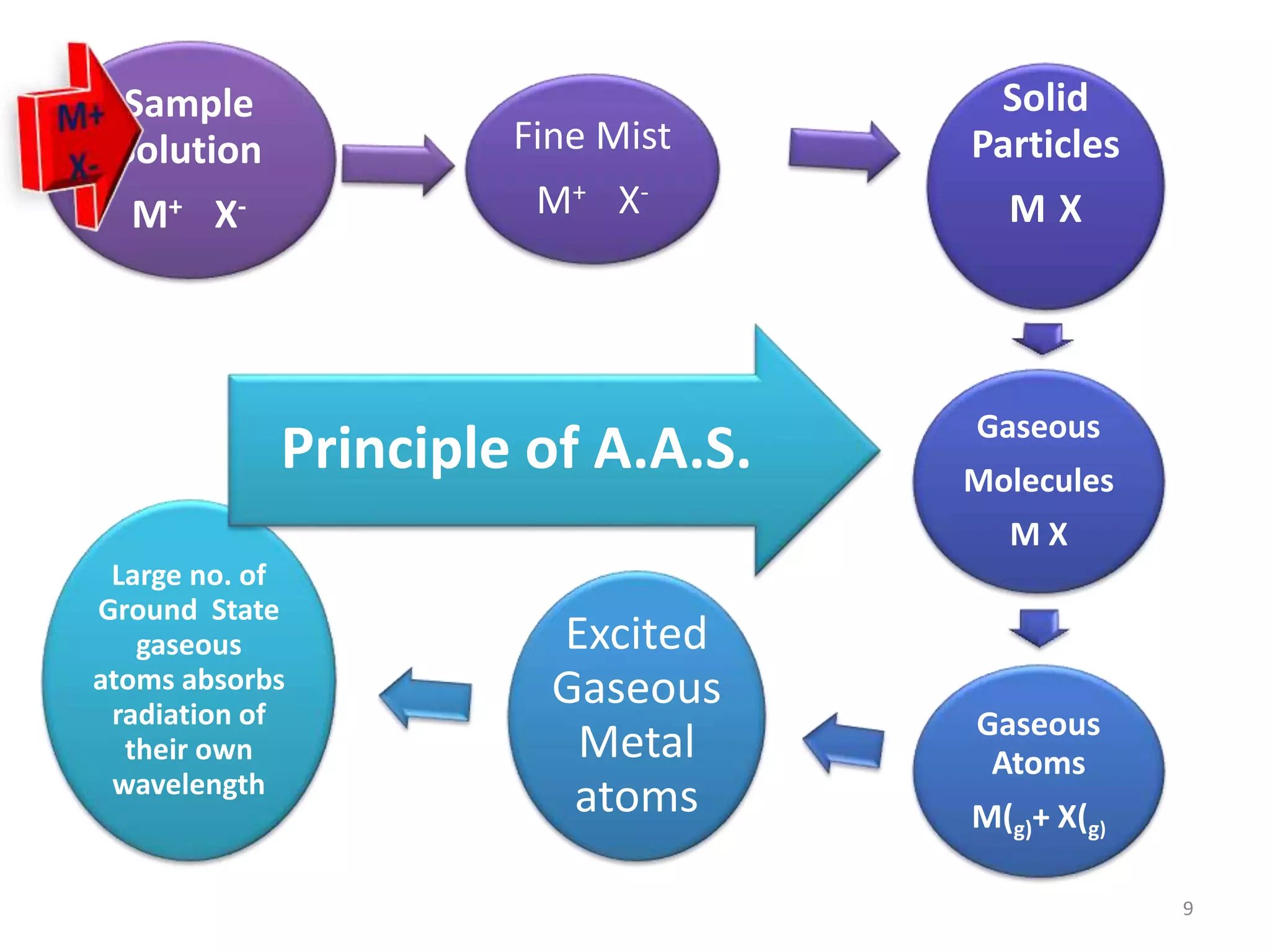
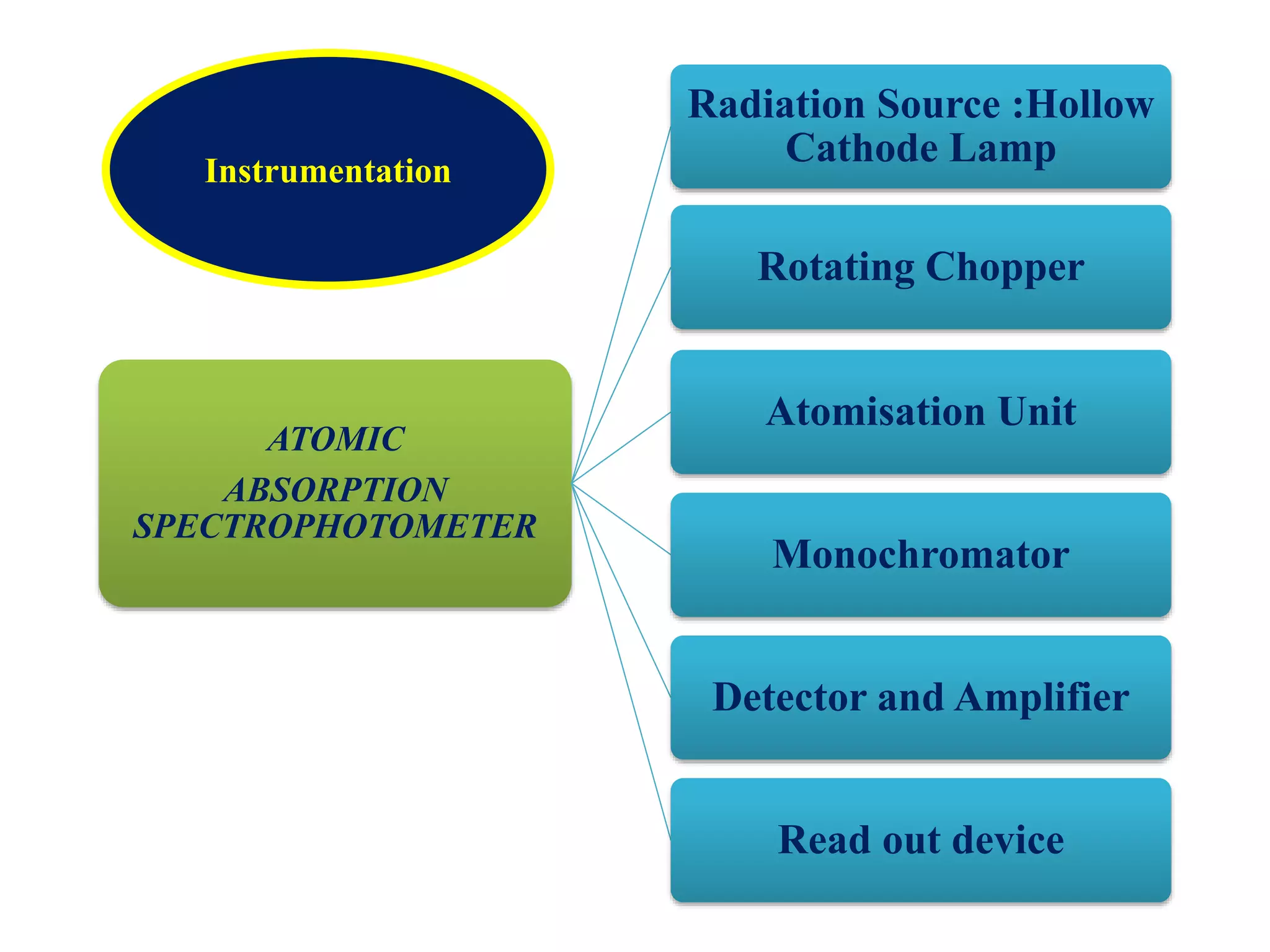
The document discusses atomic absorption spectroscopy (AAS), including its principles, instrumentation, and applications. It describes how AAS works by atomizing a liquid sample into an atomic gas using a flame or electrothermal atomizer. The instrumentation of an AAS involves a hollow cathode lamp radiation source, rotating chopper, atomization unit, monochromator, and detector. AAS provides advantages over flame emission spectroscopy such as ability to analyze various sample types and greater sensitivity using smaller sample volumes. Common applications of AAS include analysis of metals in water, foods, soils, and clinical samples.