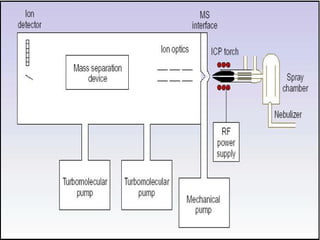
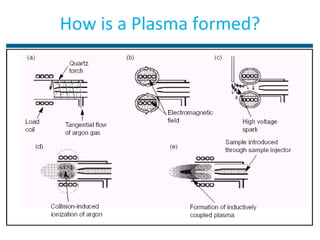
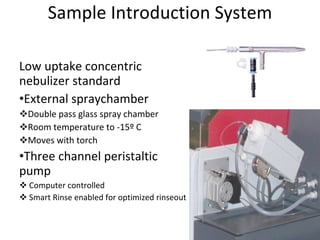
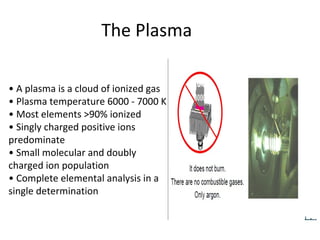
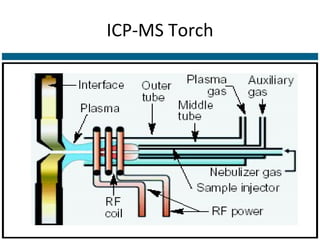
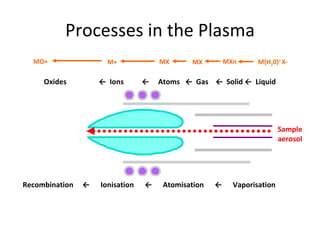
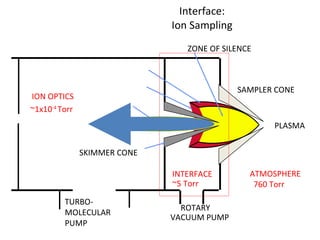
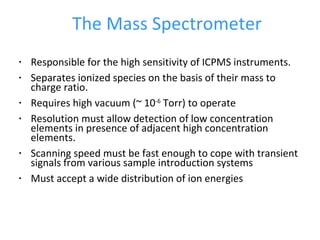
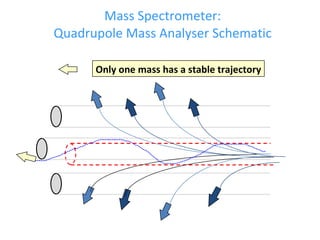
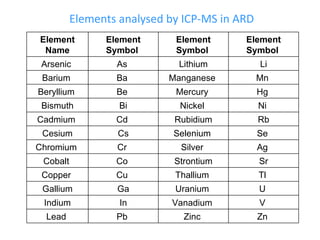
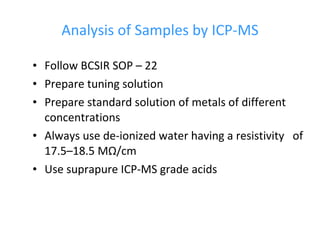
ICP-MS is an analytical technique that combines an inductively coupled plasma source with a mass spectrometer to detect elemental ions. The ICP source converts atoms in a sample to ions at very high temperatures, which are then separated and detected based on their mass-to-charge ratio in the mass spectrometer. ICP-MS provides excellent detection limits and precision for elemental analysis and can detect many elements simultaneously while also allowing for isotopic analysis. The technique requires high vacuum and specialized components to generate the plasma, transmit ions into the mass spectrometer, separate ions by mass, and detect them.