Atomic Absorption Spectrometry (Chapter 9).ppt
- 2. Introduction: • The concentration of the gaseous atoms is measured by absorption spectroscopy using Beer’s law. • From a sample in solution (used because solutions help to reduce matrix effects), generate the elemental gaseous atoms in their ground state at a concentration proportional to the concentration of the element in the sample. • There are two commonly used methods for achieving this goal: a. flame AAS b.electrothermal method.
- 3. A. Flame Atomic Absorption Spectroscopy (Flame AAS). Youtube • The most common flame method is based on a nebulizer and a slot burner. • Oxidant gas causes the nebulizer to draw up the liquid sample and produce a spray of tiny droplets. • The oxidant and fuel are pre-mixed before entering the slot burner. • The slot burner produces a linear flame 5-10 cm long.
- 4. Desirable attributes of the nebulizer and burner include: (a) Production of small & reproducible droplets. (b) No carryover effects (good drainage). (c) Production of a quiet and stable flame. (d) Prevention of flashback.
- 6. The choice of fuel and oxidant is limited. The most common combinations are: 1. acetylene - air (T = 2400 K) 2. acetylene - nitrous oxide (T = max 2800 K). Fuel/oxidant ratios can be adjusted to be 1. stoichiometric (hottest flame), 2. reducing or rich (excess fuel helps to reduce metal oxides), 3. or oxidizing or lean (excess oxidant helps to break down organics). The flame must: (a) evaporate the droplets, (b) decompose the organics and salts to atoms (c) reduce ions to atoms.
- 9. B. Electro thermal Atomization (Graphite Furnace Atomic Absorption). Use a graphite furnace - a graphite tube heated by electrical current. 1. Inject the sample (5-50 µL) into the sample cup. 2. Dry the sample (100 C). 3. Ash the sample (500-1000 C) to break down the organics. 4. Atomize the sample by ramping the temperature rapidly to 2000- 2500 C. For a fraction of a second, a plume of atoms fills the tube; the absorbance measurement must be made during this time. 5. Clean the sample cup by heating to 3000 C and then cool to room temperature. * The total time per sample is typically about 3 minutes.
- 12. Comparison of the flame and graphite furnace atomization methods: Flame: better precision, better accuracy, more rapid analyses of samples, less expensive equipment. Furnace: greater sensitivity, much lower LOD, uses much less sample.
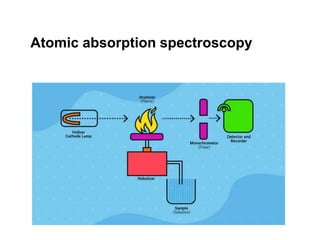
- 13. Atomic Absorption Spectrometers. Assumptions: (i) Beer's law holds for the atoms in the flame or graphite furnace, (ii) the concentration of atoms in the flame or furnace is proportional to the concentration of analyte in the sample. The block diagram:
- 15. 1. Line Source: hollow cathode lamp (HCL). • The HCL consists of a hollow cup cathode lined with the desired element and an anode in a low pressure argon atmosphere. • Application of several hundred volts causes electrons to pass through the argon, forming argon cations (Ar + ). • The Ar + ions blast atoms of the element into the gas phase and excite them by collisions. A(g) ↔ A(g)* • Consequently, the HCL emits lines of the element coating the cathode. • To change the element analyzed, one must change the HCL (although some HCL’s are coated with several elements). • Beer's law is generally valid for Abs. up to 0.8 (the upper limit of linearity in calibration curves).
- 17. 2. Chopper: Light emitted by the flame or graphite furnace (including emission by the analyte atoms) is stray light. So the HCL light is modulated by the chopper and the Amp/Processor synchronously detects the signal due to the HCL light. The chopper sends the light in a path around the flame/furnace to provide Po .
- 18. 3. Monochromator: (a) isolates one spectral line from the HCL lines (a band pass of 0.2 nm is usually sufficient to isolate 1 line); (b) prevents most of the flame or graphite furnace emission from reaching the detector. * Because the dynamic range of calibration curves is so limited (ca. 1 order-of-magnitude), it is convenient to switch to another line of the element to change the sensitivity of the method.
- 19. 4. Detector: Photomultiplier (PMT). To obtain an absorbance measurement: (a) Run a blank (e.g. pure water). The instrument records Poor adjusts PMT sensitivity to get a preset Po (b) Run a sample. The instrument calculates and displays A. Most modern instruments are automated with computer control and data acquisition.
- 20. Interferences * Chemical interferences: affect the concentration of ground state atoms in the flame/furnace. 1. Formation of stable compounds i.e. refractory oxides (WO, CaAlO2) or refractory salts (CaPO4). Possible cures: use hotter temperatures to break apart the compounds; use a rich flame to reduce metal oxides; avoid certain anions with certain analyte cations; chelate the metal analyte.
- 21. 2. Ionization (M º → M+ + e- ). Especially a problem for Group I metals which have low ionization energies. Cure: raise the free electron concentration in the gas phase by adding a large excess of Cs salt (ionization suppressant) to the sample; the excess electrons from the Cs atoms suppress ionization of other metal atoms.