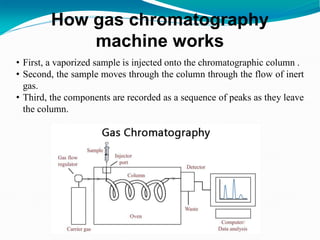
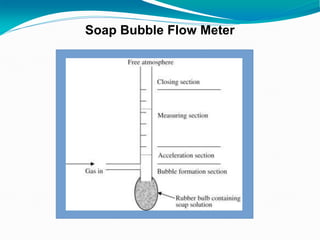
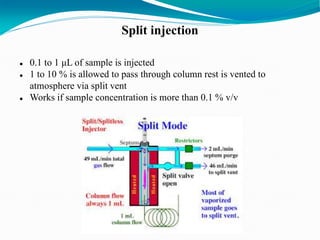
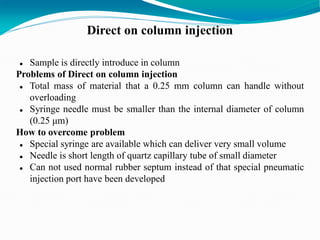
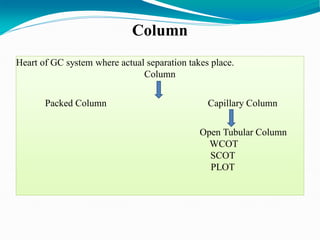
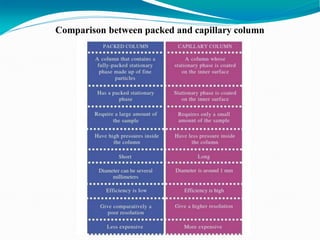
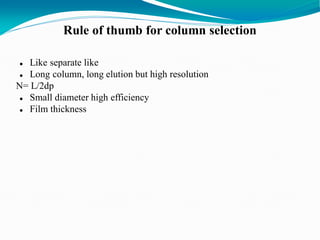
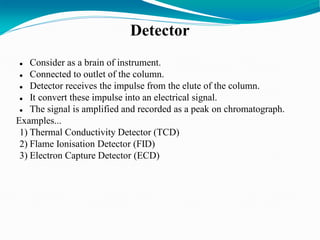
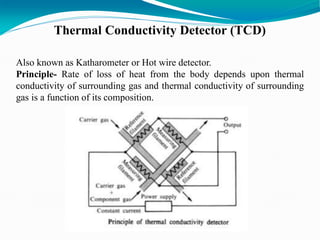
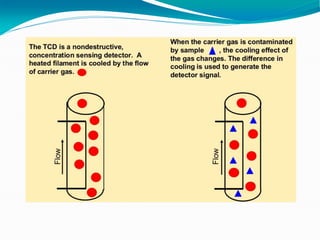
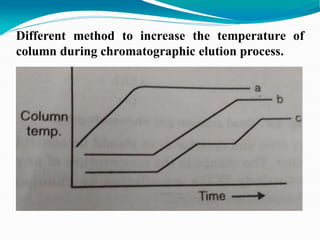
The document provides an overview of gas chromatography, detailing the principles behind gas-solid and gas-liquid chromatography, sample injection methods, and the types of columns used. It also discusses various detectors (such as TCD, FID, and ECD) and their applications in qualitative and quantitative analysis, along with the advantages and disadvantages of the technique. Additionally, it touches on programmed temperature gas chromatography to address issues with components of different boiling points.