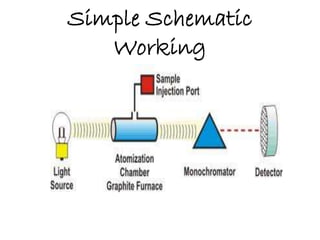
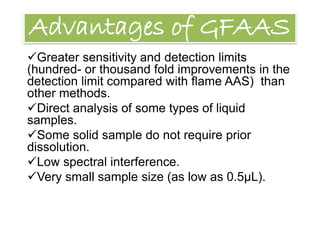
Graphite Furnace Atomic Absorption Spectroscopy (GFAAS) is a type of atomic absorption spectroscopy that uses a graphite-coated furnace to vaporize samples. Small aliquots of up to 100 microliters of aqueous samples are placed into the graphite tube and heated to high temperatures to break chemical bonds and produce free ground-state atoms. The amount of light absorbed by these atoms is proportional to the concentration of the element of interest. GFAAS offers greater sensitivity and lower detection limits than flame atomic absorption spectroscopy due to the higher temperatures achieved in the graphite furnace. It has applications in analyzing low metal concentrations in water samples and quantifying elements like beryllium in blood and serum.