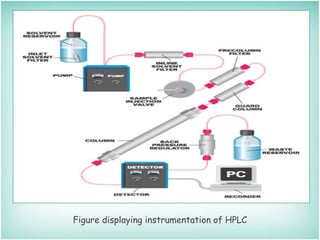
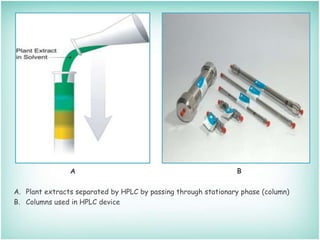
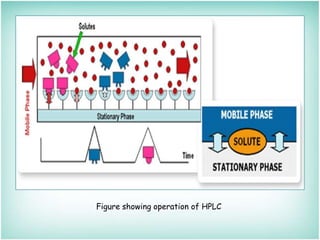
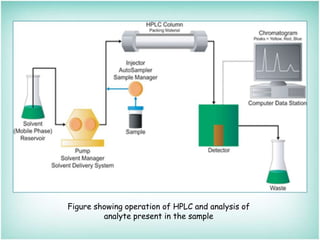
This document provides an overview of high-performance liquid chromatography (HPLC). It describes HPLC as a chromatographic technique used to separate components of a mixture for identifying, quantifying, or purifying individual components. The document outlines the history, instrumentation, operation, types, advantages, and disadvantages of HPLC. It explains that HPLC involves a mobile phase, column, pump, injector, detector, and computer to separate sample components based on their interactions with the stationary phase.